Grignard reagent is named according to its discoverer V. A. Grginard. It has very active chemical properties and is easy to react with water, carbon dioxide, alcohols, aldehydes, ketones, esters, amines and epoxy compounds, generating various types of organic compounds with the yields being generally higher. It is manufactured through the reaction between the metal magnesium and the alkyl halide in dry ether solvent, with the formula RMgX, R is an alkyl group, an aryl group or other organic group, X is chloro, bromo, iodo group such as the ether solution of the CH3MgI, C2H5MgBr, C6H5MgCl (Br) and so on. Grignard reagent is an important kind of reagents in the field of inorganic synthetic chemistry and also has wide application in the synthesis in the element organic compound. When used, we should strictly prevent contact with humid air, and pay attention to the danger of fire.
Related chemical reactions
1, the Grignard reagent can be used to have addition reaction with various kinds of unsaturated bonds, further producing different type of product. Upon reaction, the C-Mg bond in the Grignard reagent will be broken with the hydrocarbon bond being added to the atoms of low electron cloud density of the unsaturated and the magnesium atoms being added to atoms of large electron cloud density.
2, the Grignard reagent can be reacted with lead chloride. According to the different amounts of Grignard reagent and lead dichloride, it can generate dihydrocarbyl lead of tetraalkyl lead.
3, the Grignard reagent is reacted with cuprous halide to generate hydrocarbyl cuprous. Cuprous halide may be cuprous iodide, cuprous bromide and cuprous chloride.
4, the Grignard reagent reacts with dihalide mercury to generate hydrocarbyl mercury with common dihalide mercury including dibromide mercury and dichloride mercury. The reaction is carried out in an inert solvent such as ethyl ether.
5, the Grignard reagent is reacted with equal molar of zinc halide, generating hydrocarbyl zinc halide with excess amount of Grignard reagent being able to generate dihydrocarbyl zinc. Commonly used zinc halide is zinc chloride while the commonly used Grignard reagent is C2H5MgXCH2 = CHMgX, (CH3) 3CMgX and so on.
6, the Grignard reagent is reacted with a boron trihalide to generate a boron alkyl. The halogen in the Grignard reagent may be F, Cl, Br, and I. Boron trihalide is typically BF3, BCl3. The yield is 50% to 90%. We can’t use this reaction for the preparation of boron tri-t-butyl because when the t-butyl magnesium halide is reacted with boron trichloride or boron trifluoride, what we obtained in the rearrangement product -- tert-butyl diisobutyl boron or triisobutyl boron with the mechanism remaining unknown.
7, the Grignard reagent can have coupling reaction with other halogenated hydrocarbon, generating higher hydrocarbons. Commonly used halogenated hydrocarbon includes bromo-hydrocarbon and iodo-hydrocarbons with the chlorinated hydrocarbons being active enough. The reaction formula: R-MgX + X-R1 === R-R1 + MgX2.
In this reaction, the metal halides are effective catalysts. When the R = R1, people mostly use silver as catalyst; when R ≠ R1, the copper catalyst is more effective. Upon the presence of vinyl bromide, we can choose high iron catalyst. For non-active vinyl halide or aryl halides, we can use divalent-nickel dichloro-bis (triphenylphosphine) complexes or cobalt dichloride for catalysis. The catalyzed reaction process of the cobalt dichloride is as follows:

Figure 1 is a reaction scheme catalyzed by cobalt dichloride.
Preparation method
It is generally applying the reaction between metal magnesium and organic halide for generating Grignard reagent in anhydrous ether. RX + Mg = RMgX
The used raw materials and solvents must be in full dryness. Organic halides can be aliphatic or aromatic, etc., depending on the type of halogen or the whether the reaction is easy or not, the yield of Grignard reagent can also vary.
The difficulty of the reaction mainly depends on the type of hydrocarbon structure and the type of halogen in the halogenated hydrocarbon. If the hydrocarbon is the same, iodine is mostly easily to have reaction, chlorine is the mostly difficult to have reaction. However, upon using iodine, it is prone to observe Wutz side reactions and lower the yields.
If the halogen is the same, the larger the hydrocarbon group, the more difficult the reaction will be. Sometimes in order to improve the yield, we need to apply low halogen concentrations and low temperature for reactions. In order to increase the reaction rate, a small amount of iodine can be added to initiate the reaction at the beginning, once the reaction begins, because the reaction is exothermic, cooling should be rapidly performed. We can also use a small amount of 1, 2-dibromoethane to substitute iodine, especially when there is little amount of water in the diethyl ether, it is better to use of this method because the resulting magnesium bromide has a dehydrating effect.
Regarding the solvent, we may employ as diethyl ether, dibutyl ether, tetrahydrofuran and anisole. Different solvents can directly affect the yield and the level of difficulty. According to the perspective of H. Normant, the tetrahydrofuran is more appropriate, and can make the process of using vinyl chloride, chlorobenzene for preparation of Grignard reagent, which is conventionally though to be difficult, be easy. This is because of vinyl chloride and the chlorine bound to the olefinic carbon can’t react with magnesium in ether, but the reaction can occur in tetrahydrofuran, the obtained chloroethyl magnesium reagents is also known as Norman reagent. In addition to the tetrahydrofuran, we can also use 2-methyltetrahydrofuran as the solvent.
When preparing a Grignard reagent, if it was carried out in xylene, the reaction speed is very slow. However, after dropping of a small amount of ether, the reaction speed will become much faster. Therefore, it can be considered that ether acts as a catalyst.
It has also proven that, magnesium chloride can react with chlorine under pressure to direct synthesize the Grignard reagent without solvents. We can also use indirect methods to obtain it. For example, the reaction between ethyl magnesium bromine with active hydrogen compound such as acetylene can produce the displacement reaction between the metal and hydrogen, thereby obtaining bromo-ethynyl magnesium. Or it can react with magnesium bromide to displace the metal, shown in Figure 2:
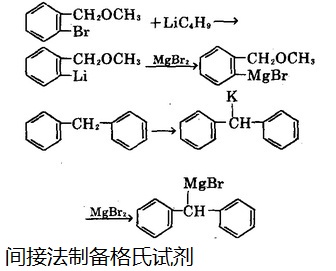
Figure 2 is an indirect preparation process of a Grignard reagent.