| Description | Phenoxybenzamine hydrochloride (dibenzyline) is an orally effective, long-acting non-selective (alpha1 and alpha2) alpha-adrenoceptor blocking agent. It is used for the control of episodes of hypertension associated withpheochromocytoma. It literally produces a "chemical sympathectomy". It increases blood flow to the skin, mucosa and abdominal viscera, and lowers both supine and erect blood pressures. It has no effect on the parasympathetic system. The initialdose is 10 mg/day, increased by 10 mg every 4 days till the desired response is attained.The usual dosage range is 20-60mg/day. |
| Chemical Properties | white crystalline solid or powder. Odorless. soluble in ethanol, chloroform, propylene glycol, slightly soluble in benzene, slightly soluble in cold water. The melting point of its free base is 38-40 ° C, and it is soluble in benzene. |
| Originator | Dibenzyline, SKF, US ,1953 |
| Uses | Phenoxybenzamine is used in treating pheochromocytoma, swelling of the medullary
layer of the adrenal glands, during which a large quantity of epinephrine is produced,
which leads to a significant elevation of blood pressure. |
| Uses | Phenoxybenzamine (5.0 to 60.0 mg/day) is used in order to control episodes of hypertension and sweating, it has a relatively slow onset and prolonged effect when compared to alternative α-blockers. If tachycardia is excessive, it may also be necessary to use a betablocker concomitantly. In addition, phenoxybenzamine has been tested for its efficacy in micturition disorders resulting from neurogenic bladder, functional outlet obstruction, and partial prostatic obstruction. |
| Definition | ChEBI: Phenoxybenzamine hydrochloride is an organic molecular entity. |
| Brand name | Dibenzyline (WellSpring). |
| General Description | Phenoxybenzamine hydrochloride is a white crystalline powder. Melting point 137.5-140°C. Used as an antihypertensive drug. |
| Air & Water Reactions | Light sensitive and may be sensitive to exposure to air . Insoluble in water. |
| Mechanism of action | Phenoxybenzamine hydrochloride is a noncompetitive alpha-adrenergicreceptor blocker, and its action cannot be nullified by increasing the amount of agonist, or agonists. It causes epinephrine reversal in that the administration of epinephrine after pretreatment with phenoxybenzamine elicits vasodilation, and, conversely, phenoxybenzamine reverses epinephrine-mediated vasoconstriction to vasodilation. It may inhibit neuronal and extraneuronal uptake mechanisms of norepinephrine. At higher concentrations, it inhibits responses to 5-HT, histamine and acetylcholine. |
| Clinical Use | Oral phenoxybenzamine is used for the preoperativemanagement of patients with pheochromocytoma and in thechronic management of patients whose tumors are notamenable to surgery. Only about 20% to 30% of an oraldose is absorbed. |
| Side effects | The adverse effects of phenoxybenzamine include nasal congestion, miosis, postural hypotension, tachycardia, and inhibition of ejaculation. |
| Safety Profile | Confumed carcinogen
with experimental carcinogenic and
teratogenic data. Poison by intraperitoneal,intravenous, and subcutaneous routes.
Human systemic effects by ingestion:
changes in tubules, including acute renal
failure, acute tubular necrosis. Moderately
toxic by ingestion. Other experimental
reproductive effects. Mutation data
reported. A long-acting adrenergic blocker.
When heated to decomposition it emits very
toxic fumes of NOx and Cl-. |
| Synthesis | Phenoxybenzamine, N-(2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)
benzylamine (12.2.5), is synthesized by reacting phenol with propylenoxide, which forms
1-phenoxy-2-propanol (12.2.1), the chlorination of which with thionyl chloride gives
1-phenoxy-2-propylchloride (12.2.2). Reacting this with 2-aminoethanol leads to formation of
1-phenoxy-2-(2-hydroxyethyl)aminopropane (12.2.3). Alkylation of the secondary amino
group gives N-(2-hydroxyethyl)-N-(1-methyl-2-phenoxyethyl)benzylamine (12.2.4), the
hydroxyl group of which is chlorinated using thionyl chloride, giving phenoxybenzamine
(12.2.5) [31]. 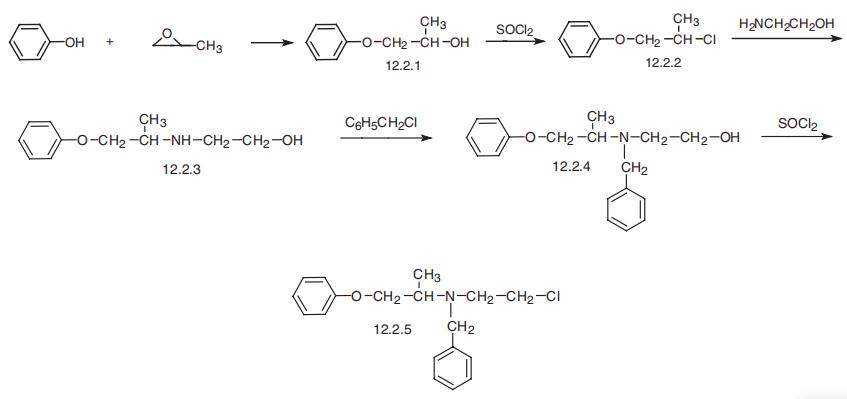
|
| Veterinary Drugs and Treatments | Phenoxybenzamine is used in small animals primarily for its effect
in reducing internal urethral sphincter tone in dogs and cats when
urethral sphincter hypertonus is present. It can also be used to treat
the hypertension associated with pheochromocytoma prior to surgery
or as adjunctive therapy in endotoxicosis.
In horses, phenoxybenzamine has been used for preventing or
treating laminitis in its early stages and to treat secretory diarrheas. |
| Drug interactions | Dibenzyline (phenoxybenzamine hydrochloride) may interact with compounds that stimulate both alpha- and beta-adrenergic receptors (i.e., epinephrine) to produce an exaggerated hypotensive response and tachycardia. |
| Carcinogenicity | Phenoxybenzamine hydrochloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. |
| Metabolism | Metabolised in the liver and excreted in the urine and bile,
but small amounts remain in the body for several days. |
| Dosage | Initially, 10 mg of Dibenzyline (phenoxybenzamine hydrochloride) twice a day. Dosage should be increased every other day, usually to 20 to 40 mg 2 or 3 times a day, until an optimal dosage is obtained, as judged by blood pressure control. |



