|
| | DICUMAROL Basic information |
| Product Name: | DICUMAROL | | Synonyms: | BIS-HYDROXYCOUMARIN;BISCUMAROL;DICUMAROL;DICOUMARIN;DICOUMAROL;2H-1-Benzopyran-2-one, 3,3'-methylenebis[4-hydroxy-;3,3’-methyleen-bis(4-hydroxy-cumarine);3,3’-methylen-bis(4-hydroxy-cumarin) | | CAS: | 66-76-2 | | MF: | C19H12O6 | | MW: | 336.3 | | EINECS: | 200-632-9 | | Product Categories: | DICUMOL | | Mol File: | 66-76-2.mol |  |
| | DICUMAROL Chemical Properties |
| Melting point | 290-292 °C(lit.) | | Boiling point | 392.79°C (rough estimate) | | density | 1.2864 (rough estimate) | | refractive index | 1.4450 (estimate) | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO:3.06(Max Conc. mg/mL);9.1(Max Conc. mM)
DMSO:PBS (pH 7.2) (1:1):0.5(Max Conc. mg/mL);1.49(Max Conc. mM)
DMF:1.25(Max Conc. mg/mL);3.72(Max Conc. mM)
Water:50.0(Max Conc. mg/mL);148.68(Max Conc. mM) | | form | Fine Crystalline Powder | | pka | 4.20±1.00(Predicted) | | color | White | | Water Solubility | Soluble in aqueous alkaline solutions, organic bases, 0.1 N NaOH (15 mg/ml), Pyridine (50 mg/ml), chloroform (slightly soluble), and benzene (slightly soluble). Insoluble in water, and alcohols. | | Merck | 14,3090 | | EPA Substance Registry System | 2H-1-Benzopyran-2-one, 3,3'-methylenebis[4-hydroxy- (66-76-2) |
| | DICUMAROL Usage And Synthesis |
| Description | The plants containing dicoumarol mainly include red carnation grass (Trifolium
pratense L., hongchezhoucao), rotten alfalfa (Medicago sativa L., zimuxu), rotten
white vanilla rhinoceros (Melilotus albus Desr., baixiangcaomuxi), and other plants
in Leguminosae. | | Description | Dicoumarol is a competitive inhibitor of NADH:quinone oxidoreductase (NQO1) with IC50 values of 2.6 and 404 nM in the absence and presence of 2 μM BSA, respectively. It has antiproliferative activity against MIA PaCa-2 pancreas and HCT116 colon carcinoma cells (IC50s = 52 and 19 μM, respectively, after a 96 hour incubation). Dicoumarol inhibits stress-activated protein kinase (SAPK) in HEK293 cells (IC50 = 19-33 μM) at a point upstream of MEKK1 and downstream of TNF receptor-associated factor 2 (TRAF2), and it inhibits TNF-α and LPS-induced NF-κB activation in HeLa cells. It also has antiproliferative activity against FL5.12 lymphocytic and MCF-7 breast carcinoma cells (100 μM) by suppressing JNK activation. | | Chemical Properties | white fine crystalline powder | | Physical properties | Appearance: white or milky white crystalline powder, slightly fragran; Solubility:
not dissolved in water, ethanol, and ether, slightly dissolved in chloroform, dissolved in alkali solution; Melting point: 287–293?°C.?It can be bluer or with purple
fluorescence in the ultraviolet light. | | History | In 1940, Karl Paul Link, a fertile scientist from the University of Wisconsin in the
United States, first isolated the anticoagulant substance from the moldy alfalfa
(Melilotus) and determined its structure. It is a kind of dicoumaroloid substance,
combined by two molecules of coumarin substances. Since this material was found
in the first few years, it has been used as a rodenticide .
In 1979, Conrad et?al. reacted p-nitrobenzene ketone with 4-hydroxycoumarin to
obtain vinegar coumarin, which is basically the same as warfarin in anticoagulant,
but its metabolites also have anticoagulant effect, so the duration of anticoagulation
is longer than warfarin. | | Uses | Dicumarol is a natural chemical used as an anticoagulant agents that functions as a vitamin K antagonist and is also a derivative of Coumarin. | | Uses | This drug is used for preventing and treating thrombosis, thrombophlebitis, thromboemolium,
and for preventing thrombo-formation in post-operational periods. | | Definition | ChEBI: A hydroxycoumarin that is methane in which two hydrogens have each been substituted by a 4-hydroxycoumarin-3-yl group. | | Indications | Intravascular thromboembolic diseases include postoperative or postoperative
thrombotic phlebitis, pulmonary embolism, myocardial infarction, and atrial fibrillation caused by embolism. | | General Description | Dicumarol, 3,3'-methylenebis[4-hydroxycoumarin],is a white or creamy white crystalline powderwith a faint, pleasant odor and a slightly bitter taste. It ispractically insoluble in water or alcohol, slightly soluble inchloroform, and dissolved readily by solutions of fixed alkalies.The effects after administration require 12 to 72 hours todevelop and persist for 24 to 96 hours after discontinuance. | | Biochem/physiol Actions | Prototype of the 4-hydroxycoumarin class of anticoagulants, which act as vitamin K antagonists, preventing formation of prothrombin. There are many reports that dicumarol also inhibits NADPH:quinone oxidoreductase (NQO(1)). In one, it inhibited NQO(1) in a pancreatic cancer cell line, causing increased formation of superoxide and inhibiting cell growth. | | Pharmacology | Dicoumarin is an oral anticoagulant drug and is invalid in?vitro . Dicoumarin is a
coumarin derivative, and its common mechanism is to inhibit synthesis of the coagulation factor in the liver. The structure of dicoumarin is similar to that of vitamin K
and is an antagonist or a competitive inhibitor of vitamin K.?It binds to the vitamin
K epoxide reductase in the liver, inhibits the conversion of vitamin K from epoxide
to hydroquinone, and inhibits the recycling of vitamin K, resulting in that the glutamate side chain of vitamin K-dependent coagulation factors II, VII, IX, and X cannot be carboxylated by γ-carboxy glutamate groups, affecting the binding of
coagulation factor with calcium ion, and thereby inhibiting coagulation, reducing
platelet adhesion, and prolonging thrombosis time . Dicoumarol drugs have no
direct confrontation with synthesized prothrombin and coagulation factor, so it is
ineffective in?vitro. After withdrawal of dicoumarin, prothrombin and coagulation
factors II, IV, IX, and X gradually restore to a certain level, and hence the anticoagulant effect disappear, so its efficacy can be maintained for a long time . | | Pharmacokinetics | Dicoumarol is not completely absorbed in the
gastrointestinal tract, often is associated with gastrointestinal discomfort, and is very rarely used
clinically. Today, the only coumarin used in the United States is warfarin, but phenprocoumon and
acenocomumarol are used in Europe. | | Clinical Use | Dicumarol is used alone or as an adjunct to heparin in theprophylaxis and treatment of intravascular clotting. It is usedin postoperative thrombophlebitis, pulmonary embolus, acuteembolic and thrombotic occlusion of peripheral arteries, andrecurrent idiopathic thrombophlebitis. It has no effect on analready-formed embolus but may prevent further intravascularclotting. Because the outcome of acute coronary thrombosisdepends largely on extension of the clot and formation ofmural thrombi in the heart chambers, with subsequent embolization,dicumarol has been used in this condition. It hasalso been administered to arrest impending gangrene afterfrostbite. The dose, after determination of the prothrombinclotting time, is 25 to 200 mg, depending on the size and thecondition of the patient. The drug is given orally in the formof capsules or tablets. On the second day and thereafter, itmay be given in amounts sufficient to maintain the prothrombinclotting time at about 30 seconds. If hemorrhages shouldoccur, a dosage of 50 to 100 mg of menadione sodium bisulfiteis injected, supplemented by a blood transfusion. | | Side effects | The most serious
adverse reaction of warfarin is bleeding, which can be against by vitamin K, and if
necessary, fresh plasma or whole blood can be injected into the body to confront
bleeding . | | Synthesis | Dicoumarol, 3,3??-methylene-bis(4-hydroxycoumarin) (24.1.8), is synthesized
from 4-hydroxycoumarine (24.1.7), which is in turn synthesized from salicylic acid
methyl ester by cyclization to a chromone derivative using sodium or sodium methoxide;
or from o-oxyacetophenone by reacting it with diethylcarbonate in the presence of sodium
ethoxide. Condensation of the resulting 4-hydroxycoumarin with formaldehyde as a phenol
component gives dicoumarol. 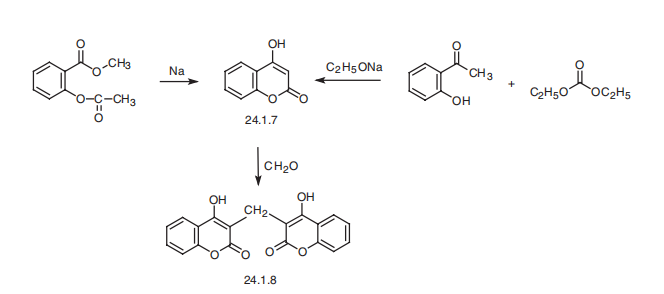
|
| | DICUMAROL Preparation Products And Raw materials |
|