| Description | Alfuzosin (SL-77499) (I), a quinazoline derivative which
is a uroselective alpha-1 adrenoreceptor antagonist, has been
developed and launched worldwide by Sanofi-Synthelabo,
for the treatment of benign prostate hyperplasia (BPH).
In November 2003, alfuzosin (I) was launched as an
extended release formulation in the US as Uroxatral utilizing
Skyepharma’s oral controlled release technology. |
| Chemical Properties | White to Off-White Solid |
| Uses | Antihypertensor;Alpha 1- adrenergic antagonist |
| Uses | a-1- Adrenoceptor antagonist structurally similar to prozosin |
| Uses | α1-Adrenoceptor antagonist structurally similar to Prozosin. Antihypertensive. Alfuzosin hydrochloride is used in treatment of benign prostatic hypertrophy.
|
| Brand name | Uroxatral (Sanofi Aventis). |
| General Description | Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. |
| Biological Activity | Functionally uro-selective α 1 adrenoceptor antagonist that does not discriminate between α 1 subtypes. Inhibits increases in intraurethral pressure caused by phenylephrine-induced contraction by 81% with minor cardiovascular effects. Also relaxes corpus cavernosum tissue (pIC 50 = 7.64) in vitro . |
| Biochem/physiol Actions | Alfuzosin hydrochloride is an alpha-adrenergic blocker used to treat benign prostatic hyperplasia (BPH). It works by relaxing the muscles in the prostate and bladder neck, making it easier to urinate. |
| Clinical Use | Alpha-blocker:
Treatment of benign prostatic hyperplasia
Treatment of acute urinary retention |
| Synthesis | Although
syntheses of alfuzosin (I) have appeared in several reports, an optimized route used for the manufacture of the compound does not appear in the literature. The synthesis
reported by the Sanofi group for alfuzosin will be described
and is shown in the scheme. The commercially available 4-
amino-2-chloro-6,7-dimethoxyquinazoline (1) was treated
with 3-methylaminopropionitrile (2) in isoamyl alcohol and
refluxed for 5 hrs. Filtration of the precipitated product and
washing with ethanol gave nitrile 3 in 62% yield.
Hydrogenation of the nitrile was done in 15% ammonia
solution in ethanol with Raney nickel as catalyst at 70??C
and 1000 psi to obtain the corresponding amine free base.
Conversion of the free base to the hydrochloride salt was
done in ethanol to give the HCl salt 4 in 52% yield. The
final acylation of amine 4 was done with the imidazolyl
anhydride of furan 5. Thus, 2-carboxyfuran was treated with
carbonyldiimidazole in THF at 40??C for 1 hr and then
cooled to 10??C. Addition of amine 4 in THF in the presence
of triethylamine at 10??C, then refluxing the reaction for 1 hr,
and aqueous workup gave the alfuzosin free base. After
conversion to the hydrochloride salt and recrystallization
from 2-propanol alfuzosin hydrochloride (I) was obtained in
44% yield. 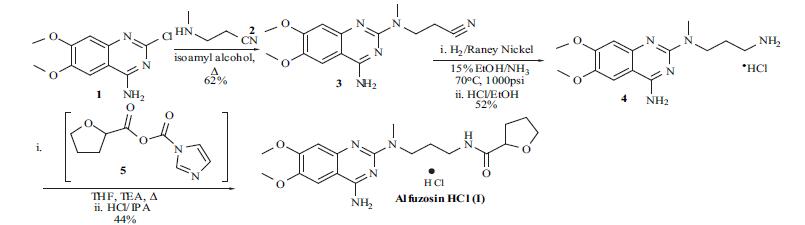
|
| Drug interactions | Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Antidepressants: enhanced hypotensive effect with
MAOIs.
Antivirals: concentration possibly increased by
ritonavir - avoid; avoid with telaprevir.
Avanafil, vardenafil, sildenafil and tadalafil: enhanced
hypotensive effect, separate administration by 4-6
hours.
Beta-blockers: enhanced hypotensive effect;
increased risk of first dose hypotensive effect.
Calcium-channel blockers: enhanced hypotensive
effect; increased risk of first dose hypotensive effect.
Cobicistat: concentration of alfuzosin possibly
increased - avoid.
Diuretics: enhanced hypotensive effect; increased
risk of first dose hypotensive effect.
Moxisylyte: possibly severe postural hypotension. |
| Metabolism | Extensively metabolised in the liver, mainly by the
cytochrome P450 isoenzyme CYP3A4, to inactive
metabolites that are mainly excreted in faeces via the bile. |



