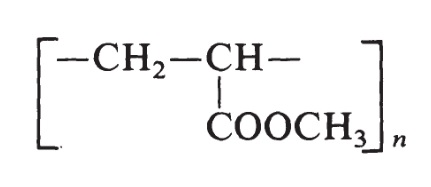|
| | POLY(METHYL ACRYLATE) Basic information |
| | POLY(METHYL ACRYLATE) Chemical Properties |
| | POLY(METHYL ACRYLATE) Usage And Synthesis |
| Chemical Properties | clear liquid | | Chemical Properties | Polymethacrylates are synthetic cationic and anionic polymers of
dimethylaminoethyl methacrylates, methacrylic acid, and
methacrylic acid esters in varying ratios. Several different types
are commercially available and may be obtained as the dry powder,
as an aqueous dispersion, or as an organic solution. A (60 : 40)
mixture of acetone and propan-2-ol is most commonly used as the
organic solvent.
Eudragit E is a cationic polymer based on dimethylaminoethyl
methacrylate and other neutral methacrylic acid esters. It is soluble
in gastric fluid as well as in weakly acidic buffer solutions (up to pH≈5). Eudragit E is available as a 12.5% ready-to-use solution in
propan-2-ol–acetone (60 : 40). It is light yellow in color with the
characteristic odor of the solvents. Solvent-free granules contain
≈98% dried weight content of Eudragit E. Eudragit E PO is a white
free-flowing powder with at least 95% of dry polymer.
Eudragit L and S, also referred to as methacrylic acid
copolymers in the USP32–NF27 monograph, are anionic copolymerization
products of methacrylic acid and methyl methacrylate.
The ratio of free carboxyl groups to the ester is approximately 1 : 1
in Eudragit L (Type A) and approximately 1 : 2 in Eudragit S (Type
B). Both polymers are readily soluble in neutral to weakly alkaline
conditions (pH 6–7) and form salts with alkalis, thus affording film
coats that are resistant to gastric media but soluble in intestinal
fluid. They are available as a 12.5% solution in propan-2-ol
without plasticizer (Eudragit L 12.5 and S 12.5); and as a 12.5% ready-to-use solution in propan-2-ol with 1.25% dibutyl phthalate
as plasticizer (Eudragit L 12.5 P and S 12.5 P). Solutions are
colorless, with the characteristic odor of the solvent. Eudragit L -
100 and Eudragit S-100 are white free-flowing powders with at
least 95% of dry polymers.
Eudragit FS 30 D is the aqueous dispersion of an anionic
copolymer based on methyl acrylate, methyl methacrylate, and
methacrylic acid. The ratio of free carboxyl groups to ester groups is
approximately 1 : 10. It is a highly flexible polymer, designed for use
in enteric-coated solid-dosage forms, and dissolves in aqueous
systems at pH >7.
Eudragit RL and Eudragit RS, also referred to as ammonio
methacrylate copolymers in the USP32–NF27 monograph, are
copolymers synthesized from acrylic acid and methacrylic acid
esters, with Eudragit RL (Type A) having 10% of functional quaternary ammonium groups and Eudragit RS (Type B) having
5% of functional quaternary ammonium groups. The ammonium
groups are present as salts and give rise to pH-independent
permeability of the polymers. Both polymers are water-insoluble,
and films prepared from Eudragit RL are freely permeable to water,
whereas, films prepared from Eudragit RS are only slightly
permeable to water. They are available as 12.5% ready-to-use
solutions in propan-2-ol–acetone (60 : 40). Solutions are colorless
or slightly yellow in color, and may be clear or slightly turbid; they
have an odor characteristic of the solvents. Solvent-free granules
(Eudragit RL 100 and Eudragit RS 100) contain 597% of the
dried weight content of the polymer.
Eudragit RL PO and Eudragit RS PO are fine, white powders
with a slight amine-like odor. They are characteristically the same
polymers as Eudragit RL and RS. They contain 597% of dry
polymer.
Eudragit RL 30 D and Eudragit RS 30 D are aqueous
dispersions of copolymers of acrylic acid and methacrylic acid
esters with a low content of quaternary ammonium groups. The
dispersions contain 30% polymer. The quaternary groups occur as
salts and are responsible for the permeability of films made from
these polymers. Films prepared from Eudragit RL 30 D are readily
permeable to water and to dissolved active substances, whereas
films prepared from Eudragit RS 30 D are less permeable to water.
Film coatings prepared from both polymers give pH-independent
release of active substance. Plasticizers are usually added to improve
film properties.
Eudragit NE 30 D and Eudragit NE 40 D are aqueous
dispersions of a neutral copolymer consisting of polymethacrylic
acid esters. The dispersions are milky-white liquids of low viscosity
and have a weak aromatic odor. Films prepared from the lacquer
swell in water, to which they become permeable. Thus, films produced are insoluble in water, but give pH-independent drug
release.
Eudragit NM 30 D is an aqueous dispersion of a neutral
copolymer based on ethyl acrylate and methyl methacrylate, and is
of identical monomer composition to Eudragit NE 30 D.
Eudragit L 30 D-55 is an aqueous dispersion of an anionic
copolymer based on methacrylic acid and ethyl acrylate. The
copolymer corresponds to USP32–NF27 methacrylic acid copolymer,
Type C. The ratio of free-carboxyl groups to ester groups is
1 : 1. Films prepared from the copolymers dissolve above pH 5.5,
forming salts with alkalis, thus affording coatings that are insoluble
in gastric media but soluble in the small intestine.
Eastacryl 30D and Kollicoat MAE 30 DP are also aqueous
dispersions of the anionic copolymer based on methacrylic acid and
ethyl acrylate. The copolymer also corresponds to USP32–NF27
methacrylic acid copolymer, Type C. The ratio of free-carboxyl
groups to ester groups is 1 : 1. Films prepared from the copolymers
dissolve above pH 5.5, forming salts with alkalis, thus affording
coatings that are insoluble in gastric media but soluble in the small
intestine.
Eudragit L 100-55 (prepared by spray-drying Eudragit L 30 D-
55) is a white, free-flowing powder that is redispersible in water to
form a latex that has properties similar to those of Eudragit L 30 D-
55.
Acryl-EZE and Acryl-EZE MP are also commercially available
as redispersible powder forms, which are designed for enteric
coating of tablets and beads, respectively. | | Uses | PMA/methylamine borane (MeAB) composites, prepared by solution blending process finds uses as a hydrogen storage material with better dehydrogenation property compared to MeAB. | | Definition | ChEBI: An acrylate macromolecule composed of repeating methoxycarbonylethylene units. | | Production Methods | Prepared by the polymerization of acrylic and methacrylic acids or
their esters, e.g. butyl ester or dimethylaminoethyl ester | | Preparation | The structure of methyl acrylate is H2C=CH-COOCH3. The monomer used to prepare poly(methyl acrylate) is produced by the oxidation of propylene. The resin is made by free-radical polymerization initiated by peroxide catalysts and has the following formula:
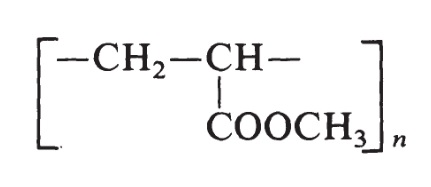
Poly(methyl acrylate) resins vary from soft, elastic, film-forming materials to hard plastics. | | Pharmaceutical Applications | Polymethacrylates are primarily used in oral capsule and tablet
formulations as film-coating agents.(1–21) Depending on the type of
polymer used, films of different solubility characteristics can be
produced;
Eudragit E is used as a plain or insulating film former. It is
soluble in gastric fluid below pH 5. In contrast, Eudragit L, S and
FS types are used as enteric coating agents because they are resistant
to gastric fluid. Different types of enteric coatings are soluble at
different pH values: e.g. Eudragit L is soluble at pH > 6 whereas
Eudragit S and FS are soluble at pH > 7. The S grade is generally
used for coating tablets, while the flexible FS 30 D dispersion is
preferred for coating particles.
Eudragit RL, RS, NE 30D, NE 40D, andNM30D are used to
form water-insoluble film coats for sustained-release products.
Eudragit RL films are more permeable than those of Eudragit RS,
and films of varying permeability can be obtained by mixing the two
types together. The neutral Eudragit NE/NM grades do not have functional ionic groups. They swell in aqueous media independently
of pH without dissolving.
Eudragit L 30 D-55 is used as an enteric coating film former for
solid-dosage forms. The coating is resistant to gastric juice but
dissolves readily at above pH 5.5.
Eudragit L 100-55 is an alternative to Eudragit L 30 D-55. It is
commercially available as a redispersible powder.
Kollicoat MAE 100 P, Acryl-EZE and Acryl-EZE MP are also
commercially available as redispersible powder forms, which are
designed for enteric coating of tablets or beads.
Eastacryl 30 D and Kollicoat MAE 30 DP are aqueous
dispersions of methacrylic acid–ethyl acrylate copolymers. They
are also used as enteric coatings for solid-dosage forms.
Polymethacrylates are also used as binders in both aqueous and
organic wet-granulation processes. Larger quantities (5–20%) of
dry polymer are used to control the release of an active substance
from a tablet matrix. Solid polymers may be used in directcompression
processes in quantities of 10–50%.
Polymethacrylate polymers may additionally be used to form the
matrix layers of transdermal delivery systems and have also been
used to prepare novel gel formulations for rectal administration. | | Safety | Polymethacrylate copolymers are widely used as film-coating
materials in oral pharmaceutical formulations. They are also used
in topical formulations and are generally regarded as nontoxic and
nonirritant materials.
Based on relevant chronic oral toxicity studies in rats and
conventionally calculated with a safety factor of 100, a daily intake
of 2–200 mg/kg body-weight depending on the grade of Eudragit
may be regarded as essentially safe in humans. | | storage | Dry powder polymer forms are stable at temperatures less than
30°C. Above this temperature, powders tend to form clumps,
although this does not affect the quality of the substance and the
clumps can be readily broken up. Dry powders are stable for at least
3 years if stored in a tightly closed container at less than 30°C.
Dispersions are sensitive to extreme temperatures and phase
separation occurs below 0°C. Dispersions should therefore be
stored at temperatures between 5 and 25°C and are stable for at
least 18 months after shipping from the manufacturer’s warehouse
if stored in a tightly closed container at the above conditions. | | Purification Methods | Precipitate it from a 2% solution in acetone by addition of water. | | Incompatibilities | Incompatibilities occur with certain polymethacrylate dispersions
depending upon the ionic and physical properties of the polymer
and solvent. For example, coagulation may be caused by soluble
electrolytes, pH changes, some organic solvents, and extremes of
temperature. For example, dispersions of Eudragit L 30
D, RL 30 D, L 100-55, and RS 30 D are incompatible with
magnesium stearate. Eastacryl 30 D, Kollicoat MAE 100 P, and
Kollicoat MAE 30 DP are also incompatible with magnesium
stearate.
Interactions between polymethacrylates and some drugs can
occur, although solid polymethacrylates and organic solutions are
generally more compatible than aqueous dispersions. | | Regulatory Status | Included in the FDA Inactive Ingredients Database (oral capsules
and tablets). Included in nonparenteral medicines licensed in the
UK. Included in the Canadian List of Acceptable Non-medicinal
Ingredients. |
| | POLY(METHYL ACRYLATE) Preparation Products And Raw materials |
|