|
| | Ticlopidine Basic information |
| Product Name: | Ticlopidine | | Synonyms: | 2-c)pyridine,4,5,6,7-tetrahydro-5-((2-chlorophenyl)methyl)-thieno(;5-((2-Chlorophenyl)methyl)-4,5,6,7-tetrahydrothieno(3,2-c)pyridine;5-(O-Chlorobenzyl)-4,5,6,7-tetrahydrothieno-[3,2-c]pyridine;PCR 5332;pcr5332;Thieno(3,2-c)pyridine, 4,5,6,7-tetrahydro-5-((2-chlorophenyl)methyl)-;Thieno[3,2-c]pyridine, 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydro-;5-(2-CHLORO-BENZYL)-4,5,6,7-TETRAHYDRO-THIENO[3,2-C]PYRIDINE | | CAS: | 55142-85-3 | | MF: | C14H14ClNS | | MW: | 263.79 | | EINECS: | 259-498-5 | | Product Categories: | | | Mol File: | 55142-85-3.mol |  |
| | Ticlopidine Chemical Properties |
| Melting point | 210-212 °C | | Boiling point | 117-120 °C(Press: 0.5 Torr) | | density | 1.273±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C(protect from light) | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | pka | 7.10±0.20(Predicted) | | form | Solid | | color | Off-White to Pale Yellow | | CAS DataBase Reference | 55142-85-3(CAS DataBase Reference) | | NIST Chemistry Reference | Thieno[3,2-c]pyridine, 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydro-(55142-85-3) | | EPA Substance Registry System | Thieno[3,2-c]pyridine, 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydro- (55142-85-3) |
| | Ticlopidine Usage And Synthesis |
| Originator | Ticlid,Millot,France,1978 | | Uses | Ticlopidine suppresses aggregation of thrombocytes and possesses antiaggregant activity. It is
believed that its action is connected to its effect on thrombocyte membranes and the reduction
in quantity of released adenosine diphosphate and serotonin, which facilitate aggregation of
thrombocytes. In wide-ranging clinical trials, ticlopidine presented a number of advantages
compared to aspirin. | | Uses | Ticlopidine is an antiplatelet drug for the treatment of ischemic heart diseases. | | Definition | ChEBI: A thienopyridine that is 4,5,6,7-tetrahydrothieno[3,2-c]pyridine in which the hydrogen attached to the nitrogen is replaced by an o-chlorobenzyl group. | | Manufacturing Process | A solution of thieno[3,2-c]pyridine (13.5 g; 0.1 mol) and 2-chlorobenzyl
chloride (17.7 g) in acetonitrile (150 ml) is boiled during 4 hours.
After evaporation of the solvent, the solid residue consists of 5-(2-
chlorobenzyl)-thieno[3,2-c]pyridinium chloride which melts at 166°C
(derivative n° 30). This compound is taken up into a solution comprising
ethanol (300 ml) and water (100 ml). Sodium borohydride (NaBH4)(20 g) is
added portionwise to the solution maintained at room temperature. The
reaction medium is maintained under constant stirring during 12 hours and is
then evaporated. The residue is taken up into water and made acidic with
concentrated hydrochloric acid to destroy the excess reducing agent. The
mixture is then made alkaline with ammonia and extracted with ether. The
ether solution is washed with water, dried and evaporated. The oily residue is
dissolved in isopropanol (50 ml) and hydrochloric acid in ethanol solution is
then added thereto.
After filtration and recrystallization from ethanol, there are obtained 5-(2-
chlorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride crystals
(yield: 60%) having a melting point (Koefler block) of 190°C. | | Brand name | 4-c-32 53-32-c;Aplaquette;Derivatives;Klodin;Opteron;Panaldine;Tcp;Ticlidan;Ticlodix;Ticlodone;Ticlopedine;Ticlosan;Tiklid;Tiklyd;Tilcid. | | Therapeutic Function | Platelet aggregation inhibitor | | World Health Organization (WHO) | Ticlopidine, an inhibitor of platelet aggregation, was introduced in
1978 for use as an antithrombotic agent. By 1982 its use had been associated with
cases of agranulocytosis, severe leucopenia and impaired haemostasis. The drug
remains available in most countries in which it was approved with appropriate
warnings in the product information. | | General Description | Ticlopidine, 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydrothieno [3,2-c]pyridine hydrochloride(Ticlid), is useful in reducing cardiac events in patients withunstable angina and cerebrovascular events in secondaryprevention of stroke. It belongs to the thienopyridine classand facilitated the development of clopidogrel. One of thedrawbacks to this agent is its side effect profile, whichincludes neutropenia, and patients receiving this antithromboticshould have their blood levels monitored. Its mechanismof action is similar to that of clopidogrel, in that itinhibits the purinergic receptors on platelets. | | Synthesis | Ticlopidine, 5-(o-chlorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
(24.2.1), is synthesized in many different ways. The first way consists of N-alkylation
of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine with 2-chlorobenzylchloride. 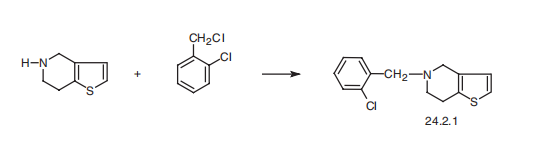
|
| | Ticlopidine Preparation Products And Raw materials |
|



