|
| | Minoxidil Chemical Properties |
| Melting point | 272-274 °C (dec.) (lit.) | | Boiling point | 348.61°C (rough estimate) | | density | 1.1651 (rough estimate) | | refractive index | 1.7610 (estimate) | | storage temp. | 2-8°C | | solubility | Slightly soluble in water, soluble in methanol and in propylene glycol. | | pka | 4.61(at 25℃) | | form | neat | | color | White | | Water Solubility | Soluble in water (2.2 mg/ml), 100% ethanol (29 mg/ml), propylene glycol, acetone, DMSO (6.5 mg/ml), and methanol. | | Merck | 14,6203 | | BCS Class | 3 | | Stability: | Stable for 2 years from date of purchase as supplied. Protect from moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | | InChIKey | ZFMITUMMTDLWHR-UHFFFAOYSA-N | | CAS DataBase Reference | 38304-91-5(CAS DataBase Reference) | | NIST Chemistry Reference | Minoxidil(38304-91-5) |
| | Minoxidil Usage And Synthesis |
| an antihypertensive vasodilator medication | Minoxidil is an antihypertensive vasodilator medication also claiming to slow or stop hair loss and promote hair regrowth. It is available over the counter for treatment of androgenic alopecia, among other baldness treatments, but measurable changes, if experienced, disappear within months after discontinuation of treatment.
Minoxidil was first sold as a drug for high blood pressure and was noted to have the interesting side-effect of increased body hair-growth, or in some cases, significant hair loss. Treatments for baldness and hair loss usually include a 5% concentration solution that is designed for men, whereas the 2% concentration solutions are designed for women.
| | Indications and Usage | Minoxidil is a type of oral drug that is clinically used to lower blood pressure, and it is also called Loniten and piperidine diamine. Minoxidil is an antihypertensive drug that is clinically used to treat intractable, primary or renal hypertension. It can also be used to treat severe hypertension that does not respond well to other antihypertensive drugs, but it needs to be used in combination with diuretic drugs to prevent water and sodium retention, and combined use with beta receptor blockers may increase its curative effects and reduce adverse effects. Minoxidil is also used to prevent grease-type alopecia and on livestock.
| | Mechanisms of Action | Minoxidil is a type of potassium channel opener and can directly relax smooth vascular muscles and has a strong small artery dilating effect. It can reduce peripheral resistance, dilate blood vessels, and lower blood pressure while having no effect on blood volume, thus promoting venous return. In addition, due to reflective regulation and positive frequency effects, it can increase cardiac output and heart rate, but will not cause orthostatic hypotension.
| | Adverse reactions | Long term use of Minoxidil may have the side effect of increased body hair, such as arm hair.
Adverse reactions to oral intake mainly include: weight gain and lower body swelling due to water and sodium retention, heart palpitations and arrhythmia due to reflexive sympathetic nervous excitement, and hirsuitism.
Adverse reactions to external use mainly include: skin irritation, red spots in applied areas, itching, and other skin inflammation reactions. Although only a small amount is absorbed, but there is also a slight possibility of causing a recurrence in patients with a history or heart disease. Use with caution if suffering from cerebrovascular disease, non-hypertensive heart failure, coronary heart disease, angina pectoris, myocardial infarction, pericardial effusion, renal dysfunction and other diseases.
| | Contradictions | Do not use if allergic to Minoxidil or suffering from pheochromocytoma.
| | Description | Minoxidil is a peripheral vasodilator that directly relaxes vascular smooth musculature,
thus, lowering systolic and diastolic pressure. Its action is linked to the activation of calcium
channels. Open calcium channels cause hyperpolarization of smooth muscle cells,
which in turn, reduces the flow of calcium ions into the cell, which is necessary for supporting
vascular tonicity. However, when taking minoxidil, tachycardia, elevated renin
secretion, and water and sodium ion retention all appear simultaneously with hypotension. | | Chemical Properties | White Crystalline Solid | | Originator | Loniten ,Upjohn ,US ,1979 | | Uses | Used as an antihypertensive and antialopecia agent. Minoxidil activates ATP-activated K+ channels | | Uses | A selective ATP dependent K+ (Kir6) channel activator | | Definition | ChEBI: Minoxidil is a pyrimidine N-oxide that is pyrimidine-2,4-diamine 3-oxide substituted by a piperidin-1-yl group at position 6. It has a role as a vasodilator agent and an antihypertensive agent. It is a pyrimidine N-oxide, a member of piperidines and an aminopyrimidine. | | Manufacturing Process | Barbituric acid is reacted with phosphorus oxychloride then with 2,4,6-trichloropyrimidine and that product with ammonia to give 4-chloro-2,6-diaminopyritnidine.
A 30 g (0.15 mol) quantity of 4-chloro-2,6-diaminopyrimidine is dissolved in600 ml of hot 3A alcohol, the solution cooled to 0°C to 10°C and 41.8 g (0.24mol) of m-chloroperbenzoic acid is added. The mixture is held at 0°C to 10°Cfor 4 hours and filtered. The solid is shaken for 2 hours in 0.24 mol of 10%sodium hydroxide and filtered. The solid is washed with water and dried toyield 193 g of crude product. This product is extracted for 1 hour with 900 mlof boiling acetonitrile to yield 14.8 g (44.7% yield) of 6-amino-4-chloro-1,2-dihydro-1-hydroxy-2-iminopyrimidine, melting point 193°C.
A mixture of 3.0 g (0.019 mol) of 6-amino-4-chloro-1,2-dihydro-1-hydroxy-2-iminopyrimidine and 35 ml of piperidine is refluxed for 1.5 hours, cooled andfiltered. The solid is shaken for 20 minutes in a solution of 0.8 g of sodiumhydroxide in 30 ml of water and filtered. The solid is washed with water andextracted with 800 ml of boiling acetonitrile and filtered to yield 3.5 g (89%)yield of 6-amino-4-chloro-1,2-dihydro-1-hydroxy-2-iminopyrimidine, meltingpoint 248°C, decomposition at 259°C to 261°C. | | Brand name | Loniten (Pharmacia & Upjohn); Rogaine (Pharmacia
& Upjohn); Minodyl (Quantum Pharmics). | | Therapeutic Function | Antihypertensive | | General Description | Minoxidil, 2,4-diamino-6-piperidinopyrimidine-3-oxide . It is converted to minoxidil sulfate in the liver bya sulfotransferase enzyme.
The antihypertensive properties of minoxidil are similarto those of hydralazine hydrochloride, in that minoxidil candecrease arteriolar vascular resistance. Minoxidil exerts itsvasodilatory action by a direct effect on arteriolar smoothmuscle and appears to have no effect on the CNS or on theadrenergic nervous system in animals. The serum half-lifeis 4.5 hours, and the antihypertensive effect may last up to24 hours. | | Biological Activity | Antihypertensive. Antialopecia agent. K + channel (K ATP ) activator. | | Biochem/physiol Actions | Activates ATP-activated K+ channels; vasodilator; slow or stop hair loss and promote hair regrowth. | | Mechanism of action | Potassium channel openers are drugs that activate (i.e., open) ATP-sensitive K+
channels in the VSM. By
opening these potassium channels, there is increased efflux of potassium ions from the cells, causing hyperpolarization
of VSM, which closes the voltage-gated calcium channels and, thereby, decreases intracellular calcium. With less
calcium available to combine with calmodulin, there is less activation of MLCK and phosphorylation of myosin light
chains. This leads to relaxation and vasodilation. Because small arteries and arterioles normally have a high degree of
smooth muscle tone, these drugs are particularly effective in dilating these resistance vessels, decreasing systemic
vascular resistance, and lowering arterial pressure. The fall in arterial pressure leads to reflex cardiac stimulation
(baroreceptor-mediated tachycardia).
Minoxidil, as its active metabolite minoxidil O-sulfate, prolongs the opening of the potassium channel, sustaining
greater vasodilation on arterioles than on veins. The drug decreases blood pressure in both the supine and standing
positions, and there is no orthostatic hypotension. Associated with the decrease in peripheral resistance and blood
pressure is a reflex response that is accompanied by increased heart rate, cardiac output, and stroke volume, which
can be attenuated by the coadministration of a β-blocker. Along with this decrease in peripheral resistance is
increased plasma renin activity and sodium and water retention, which can result in expansion of fluid volume, edema,
and congestive heart failure. The sodium- and water-retaining effects of minoxidil can be reversed by coadministration
of a diuretic. When minoxidil is used in conjunction with a β-adrenergic blocker, pulmonary artery pressure remains
essentially unchanged. | | Pharmacokinetics | Minoxidil is absorbed from the GI tract and is metabolized to its active sulfate metabolite. Plasma concentrations for
minoxidil sulfate peak within 1 hour and then decline rapidly. Following an oral dose of minoxidil, its hypotensive effect
begins in 30 minutes, is maximal in 2 to 8 hours, and persists for approximately 2 to 5 days. The delayed onset of the
hypotensive effect for minoxidil is attributed to its metabolism to its active metabolite. The drug is not bound to plasma
proteins. The major metabolite for minoxidil is its N-O-glucuronide, which unlike the sulfate metabolite is inactive as a
hypotensive agent. Approximately 10 to 20% of an oral dose of minoxidil is metabolized to its active metabolite,
minoxidil O-sulfate, and approximately 20% of minoxidil is excreted unchanged. | | Clinical Use | Minoxidil is used for severe hypertension that is difficultto control with other antihypertensive agents. The drug hassome of the characteristic side effects of direct vasodilatorydrugs. It causes sodium and water retention and may requirecoadministration of a diuretic. Minoxidil also causes reflextachycardia, which can be controlled by use of a -adrenergicblocking agent.
Minoxidil topical solution is used to treat alopecia androgenitica(male-pattern baldness). Although the mechanismis not clearly understood, topical minoxidil is believed to increasecutaneous blood flow, which may stimulate hairgrowth. The stimulation of hair growth is attributed to vasodilationin the vicinity of application of the drug, resultingin better nourishment of the local hair follicles. | | Side effects | Adverse reactions include local irritation and contact dermatitis.
If the treatment is discontinued, clinical regression occurs within 3 months
to the state of hair thinning that would have occurred had the treatment
not been started. Twice-daily treatment is more efficacious than once-daily
application. Women who use the5%concentrationmay note the development
of facial hair, which is reversible on discontinuation of the medication. The
5% concentration can be more irritating than the 2% solution. | | Synthesis | Minoxidil, 6-amino-1,2-dihydro-1-hydroxy-2-imino-4-piperidinopyrimidine
(22.8.5), is synthesized from barbituric acid, the reaction of which with phosphorous oxychloride
gives 2,4,6-trichloropyrimidine (22.8.1). Upon reaction with ammonium, this
turns into 2,4-diamino-6-chloropyrimidine (22.8.2). Next, the resulting 2,4-diamino-6-
chloropyrimidine (22.8.2) undergoes reaction with 2,4-dichlorophenol in the presence of
potassium hydroxide, giving 2,4-diamino-6-(2,4-dichlorophenoxy)-pyrimidine (22.8.3).
Oxidation of this product with 3-chloroperbenzoic acid gives 2,4-diamino-6-(2,4-
dichlorophenoxy)pyrimidine-3-oxide (22.8.4), the 2,4-dichlorophenoxyl group of which
is replaced with a piperidine group at high temperature, giving minoxidil (22.8.5). 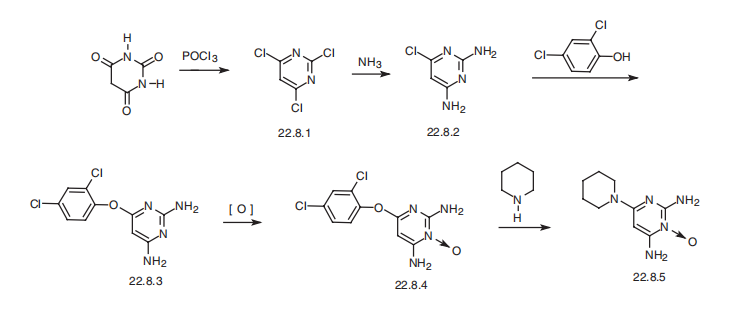
| | Drug interactions | When minoxidil is administered with diuretics or other hypotensive drugs, the hypotensive effect of minoxidil increases,
and concurrent use may cause profound orthostatic hypotensive effects. | | Metabolism | Extensively metabolised by the liver. It requires
sulphation to become active, but the major metabolite is a
glucuronide conjugate.
Excreted mainly in the urine in the form of metabolites.
Minoxidil and its metabolites are dialysable, although the
pharmacological effect is not reversed. | | storage | Room temperature | | References | 1) Meisheri et al. (1988), Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability; J. Pharmacol. Exp. Ther., 245 7
2) Messenger and Rundegren (2004) Minoxidil:mechanisms of action on hair growth; Br. J. Dermatol., 150 186 |
| | Minoxidil Preparation Products And Raw materials |
|



