|
| | Sertaconazole nitrate Basic information |
| | Sertaconazole nitrate Chemical Properties |
| Melting point | 146-147° | | Boiling point | 614.1±55.0 °C(Predicted) | | density | 1.43±0.1 g/cm3(Predicted) | | storage temp. | Refrigerator | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 6.68±0.12(Predicted) | | color | White to Pale Yellow | | CAS DataBase Reference | 99592-32-2(CAS DataBase Reference) |
| | Sertaconazole nitrate Usage And Synthesis |
| Description | Sertaconazole has been developed and launched for the
treatment of dermatological fungal infections by Ferrer
Internacional S. A. Mylan received FDA approval for
sertaconazole nitrate cream for the treatment of athlete's foot
(tinea pedis) at the end of 2003. | | Uses | An imidazole antifungal agent, inhibits the synthesis of ergosterol, an essential cell wall component of fungi. | | Definition | ChEBI: 1-{2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}imidazole is a member of the class of imidazoles that carries a 2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl group at position 1. It is a dichlorobenzene, an ether, a member of imidazoles and a member of 1-benzothiophenes. | | Synthesis | 2,4-Dichloro acetophenone
169 was brominated at low temperature to give bromide
intermediate 170, which was used without isolation. To the
same pot, five-fold excess of imidazole was added to give
imidazolylacetophenone 171 in 71% yield from 169.
Sodium borohydride was employed to reduce ketone 171 to
alcohol 172 in 78% yield. Racemic alcohol 172 was resolved with (-)-DIP-chloride to give its corresponding
chiral R-alcohol 173 in 80% yield. Compound 173 was then
alkylated with 3-bromomethyl-7-chlorobenzo[b]thiophene
(174) in dry DMF in the presence of potassium t-butoxide to
give the alkylation product in 68% yield. Finally, 60%
nitric acid was used to make sertaconazole mononitrate
(XXI) in 89% yield. 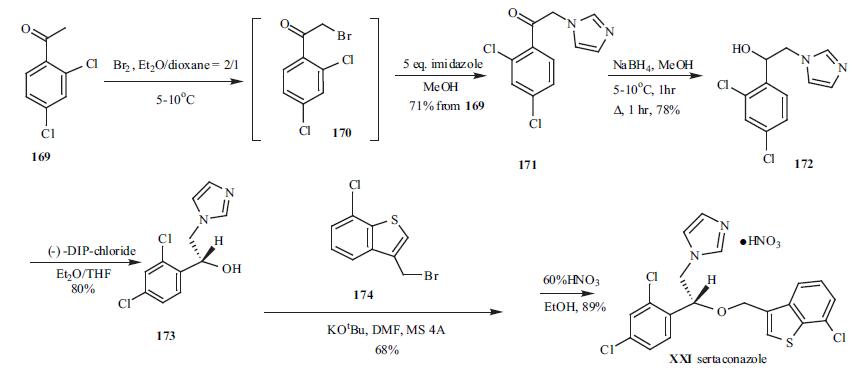
|
| | Sertaconazole nitrate Preparation Products And Raw materials |
|



