|
| Product Name: | Riociguat | | Synonyms: | Riociguat;N-[4,6-Diamino-2-[1-[(2-fluorophenyl)methyl]-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-N-methylcarbamic acid methyl ester;Bay 63-2521;Carbamic acid, (4,6-diamino-2-(1-((2-fluorophenyl)methyl)-1H-pyrazolo(3,4-B)pyridin-3-yl)-5-pyrimidinyl)methyl-, methyl ester;Riociguat (BAY 63-2521);Riocguat;CS-718;Riociguat, BZY 632521 | | CAS: | 625115-55-1 | | MF: | C20H19FN8O2 | | MW: | 422.42 | | EINECS: | 641-755-1 | | Product Categories: | API | | Mol File: | 625115-55-1.mol |  |
| | Riociguat Chemical Properties |
| Melting point | 247-251°C (dec.) | | Boiling point | 567.2±50.0 °C(Predicted) | | density | 1.51 | | storage temp. | Refrigerator | | solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) | | pka | 1.51±0.50(Predicted) | | form | Solid | | color | Off-White to Orange |
| | Riociguat Usage And Synthesis |
| Pharmacological effects | Riociguat is a soluble guanylyl cyclase activator, developed by the German Bayer ,it is an important signaling enzyme, which can be activated by nitric oxide (NO) to catalyze guanosine triphosphate ( GTP) to convert into the second messenger cyclic guanosine monophosphate (cGMP). Soluble guanylate cyclase is currently the only known NO receptor. Damage of NO-sGC-cGMP signal pathway is believed to be the cause of the incidence of cardiovascular, lung, endothelium, kidney and liver diseases. NO synthesis in patients with pulmonary hypertension is not enough, NO donor drugs, although effective, short half-life, riociguat can directly activate sGC, stable NO-sGC combination so as to upregulate the second messenger cGMP. riociquat can significantly increase exercise tolerance in patients with hemodynamic parameters of cardiac function and prolong the time to reach clinical worsening (PATENT study), It may also be effective in treating CTEPH(CHEST study) .
October 8, 2013, the US FDA approved Riociguat for the treatment of chronic thromboembolic pulmonary hypertension and PAH (Pulmonary Arterial Hypertension).
At present, Riociguat is not yet sold in the domestic market ,in the United States it is sold by the Haoeyou Pharmacy , the pharmacy is subsidiary of California Healthcom Group, because the market ofRiociguat is in its infancy, its sales grow slowly.
| | Clinical evaluation | In clinical trials ,261 patients with chronic thromboembolic pulmonary hypertension (CTEPH) were divided into riociguat and placebo groups, a six-minute walk distance (6MWD) as the main clinical endpoint, after 16 weeks of treatment, 6MWD of riociguat group increased by 46 meters higher than the average in the placebo group. 443 cases of PAH were divided into riociguat and placebo groups,after 12 weeks of treatment, riociguat group increased 36 meters higher than the average 6MWD in the placebo group. Boxed warning suggests that the drug has a embryo toxicity, pregnant patients are forbidden.
Adempas is granted baseing on two global III studies CHEST-1 and PATENT-1, whose findings are published in the July 25, 2013"New England Journal of Medicine," (2013; 369: 330-40; 2013; 369: 319-29). Thomson Reuters released analysis report that it is expected that sales of the drug in 2017 will reach $ 679 million, while the market will face a potential threat constituted by the Swiss company Actelion bosentan and Gilead drug Imber bosentan tablets.
The above information is edited by the chemicalbook of Tian Ye.
| | Production Method | Synthetic key of Riociguat is the synthesis of three heterocyclic ring. Compounds 1 and 2 directly close the pyrazole ring to generate 3,3 and 4 and then close the pyrazole ring to obtain5, ethyl of 5 is dehydrated using trifluoroacetic anhydride to give a cyano group after amidation by the ammonia , the cyano group is treated with methanol sodium and chloride ammonium to get amidine 6. The two nitrogen atoms on Amidine 6 as dinucleophile, with two cyano on compound 7 as the electrophile ,directly close the pyrimidine ring to give 8. pyrimidine 5-position amino group on 8 with a strong nucleophilic, directly reacts with methyl chloride and then methylates to give riociguat.

Figure 1 is a chemical reactions road map of Riociguat production .
| | Patent cases | US7173037 (there is a deadline to April 25, 2023), US6743798 (valid until July 16, 2019).
| | Description | In September 2013, Health Canada approved riociguat (also referred to as BAY 63-2521), for the treatment of patients with chronic thromboembolic pulmonary hypertension (CTEPH) after surgical treatment or inoperable CTEPH and for the treatment of adults with pulmonary arterial hypertension (PAH). Riociguat has a dual mode of action and works by (a) sensitizing sGC to the body’s NO by stabilizing NO–sGC binding and (b) an NO-independent, direct stimulation of sGC via a different binding site. This process restores the NO–sGC–cGMP pathway and leads to increased generation of cGMP with subsequent vasodilation.
Headache, dizziness, dyspepsia/gastritis, nausea, diarrhea, hypotension, vomiting, anemia, gastroesophageal reflux, and constipation were the most common adverse events ( 3%) observed during riociguat clinical trials. Riociguat comes with a black box warning for embryo-fetal toxicity. | | Chemical Properties | Brownish Orange Solid | | Originator | Bayer (Germany) | | Uses | Riocguat is used in the treatment for pulmonary hypertension. | | Uses | Riociguat is used in the treatment for pulmonary hypertension. | | Definition | ChEBI: A carbamate ester that is the methyl ester of {4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}methylcarbamic acid. It is used for treatment of chronic thromboembolic pulmonary hypertension an
pulmonary arterial hypertension | | Brand name | Adempas | | Clinical Use | Guanylate cyclase stimulator:
Treatment of chronic thromboembolic pulmonary
hypertension (CTEPH) and pulmonary arterial
hypertension (PAH) | | Synthesis | The sequence began with condensation of commercial 2-fluorobenzylhydrazine
(136) with sodium ethyl cyanopyruvate (137),
which derives from diethyl oxalate to generate aminopyrazole
138. This was followed by the cyclocondensation with 3-dimethylaminoacrolein
(139) to access pyrazolopyridine 140 in 50% yield
for the two-step operation. Next, ester 140 was transformed to
the corresponding primary amide 141, which was subsequently
dehydrated upon treatment with trifluoroacetic acid anhydride
(TFAA) to construct nitrile 142 in quantitative yield from 140.
Subjection of cyanopyrazole 142 to Pinner conditions using
methoxide and ammonium chloride in refluxing acetic acid generated
amidine 143, and this was followed by condensation with the
malononitrile derivative 144 in base to provide pyrimidine 145 in
73% yield. Hydrogenative cleavage of the phenyldiazine converted 145 to the pyrimidyl triamine 146, which underwent carbamoylation
at the 40 position to produce the penultimate carbamate
147. This carbamate was then selectively methylated
through deprotonation of the carbamate N¨CH proton followed by
quench with methyl iodide. Sequential recrystallization from
warm DMSO and refluxing ethyl acetate produced riociguat (XIX)
in 64% yield from 147. 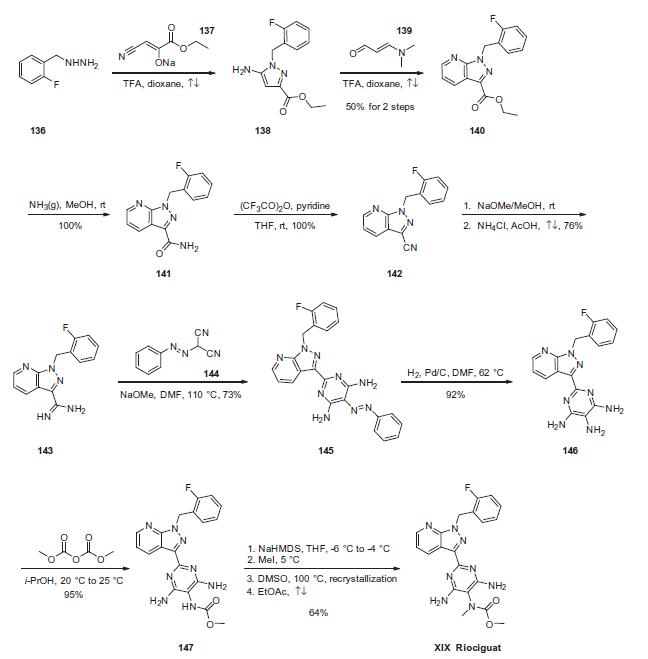
| | Drug interactions | Potentially hazardous interactions with other drugs
Avanafil, sildenafil, tadalafil, vardenafil: enhanced
hypotensive effect - avoid.
Nicorandil: possibly enhanced hypotensive effect -
avoid.
Nitrates: possibly enhanced hypotensive effect -
avoid. | | Metabolism | N-demethylation, catalysed by CYP1A1, CYP3A4,
CYP2C8 and CYP2J2 is the major biotransformation
pathway leading to its major circulating active
metabolite M-1 (pharmacological activity: 1/10th to
1/3rd of riociguat) which is further metabolised to the
pharmacologically inactive N-glucuronide.
Riociguat and metabolites are excreted via both
renal (33-45%) and biliary/faecal routes (48-59%).
Approximately 9-44% of the administered dose was
found as unchanged riociguat in faeces. |
| | Riociguat Preparation Products And Raw materials |
|