|
| | Verapamil Basic information |
| Product Name: | Verapamil | | Synonyms: | -2-isopropyl-;2-isopropyl-;4-dimethoxy-alpha-(1-methylethyl)-l)-;5-((3,4-dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropylv;Benzeneacetonitrile, .alpha.-3-2-(3,4-dimethoxyphenyl)ethylmethylaminopropyl-3,4-dimethoxy-.alpha.-(1-methylethyl)-;Benzeneacetonitrile, a-[3-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]propyl]-3,4-dimethoxy-a-(1-methylethyl)- (9CI);dl-Verapamil;NSC 272306NA | | CAS: | 52-53-9 | | MF: | C27H38N2O4 | | MW: | 454.61 | | EINECS: | 200-145-1 | | Product Categories: | Verapamil | | Mol File: | 52-53-9.mol |  |
| | Verapamil Chemical Properties |
| Melting point | 25°C | | Boiling point | 243-246 °C (1.3 Pa) | | density | 1.1267 (rough estimate) | | refractive index | 1.5448 | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | DMSO: 100 mg/mL (219.97 mM) | | pka | 8.6(at 25℃) | | form | Thick Oil | | color | Colourless | | CAS DataBase Reference | 52-53-9(CAS DataBase Reference) | | NIST Chemistry Reference | Verapamil(52-53-9) | | EPA Substance Registry System | Verapamil (52-53-9) |
| | Verapamil Usage And Synthesis |
| Originator | Isoptin,Knoll ,W. Germany ,1963 | | Uses | Vasodilator (coronary). | | Uses | Verapamil is primarily used as an antiarrythmic for treating ventricular
arrhythmias; however, currently it is being forced out gradually by adenosine. | | Uses | Verapamil is used for preventing angina pectoris attacks, arterial hypertension, and treating
and preventing supraventricular arrhythmia (paroxysmal supraventricular tachycardia,
atrial fibrillation, atrial flutter, extrasystole). | | Definition | ChEBI: A tertiary amino compound that is 3,4-dimethoxyphenylethylamine in which the hydrogens attached to the nitrogen are replaced by a methyl group and a 4-cyano-4-(3,4-dimethoxyphenyl)-5-methylhexyl group. | | Manufacturing Process | 177.2 g (1 mol) of veratryl cyanide are dissolved in 1 liter of toluene in a
three-neck flask. 42.9 g (1.1 mols) of pulverized sodium amide are added.
The mixture is heated to boiling under reflux for one hour while stirring and
excluding moisture. A solution of the base (N-methyl-N-homoveratryl)-γ-
aminochloropropane, freshly prepared from 339.2 g (1.1 mols) of the
hydrochloride, in 1.2 liters of toluene is added drop by drop into this boiling
mixture within two hours while stirring vigorously. Heating and stirring are
continued for four more hours. After cooling, the reaction mixture is poured
into 3 liters of ice water while stirring, The mixture is acidified with 20%
hydrochloric acid. The acidified aqueous layer is separated, neutralized by the
addition of sodium hydroxide solution, and rendered alkaline by the addition
of concentrated potassium carbonate solution. The precipitated oily base is
taken up in benzene. On evaporating the solvent, 402 g of the crude base are
obtained in the form of a reddish-brown, viscous oil.
The crude base is dissolved in a mixture of 550 ml of isopropanol and 650 ml
of ethyl acetate; Gaseous hydrogen chloride is introduced into the solution
until it is of weakly acidic reaction. On allowing the mixture to stand at 0°C,
365 g of α-[(N-methyl-N-homoveratryl)-γ-amino-propyl]-3,4-dimethoxyphenyl
acetonitrile hydrochloride precipitate as a slightly yellowish crystal powder of
the melting point 136°C to 139°C (corr.). Yield: 81% of the theoretical yield.
The pure, white hydrochloride melting at 140°C to 142°C (corr.) is obtained
on recrystallizing the crude salt twice from isopropanol with the addition of
decolorizing carbon. The salt is very soluble in water. The base prepared from
the hydrochloride in the form of an almost colorless, very viscous oil boils at
233°C to 235°C/0.01 mm Hg; nD25= 1.5532. Dioxalate, melting point: 123°C
to 125°C (corr.), on recrystallization from acetone and isopropanol.
61.9 g (0.15 mol) of α-[(N-methyl-N-homoveratryl)-γ-aminopropyl]-3,4-
dimethoxyphenyl acetonitrile are dissolved in 300 ml of toluene. The solution
is heated to boiling under reflux with 8.5 g (1.45 x 0.15 mols) of pulverized
sodium amide for one hour while stirring. Thereafter, a solution of 31.4 g (1.7
x 0.15 mols) of isopropyl bromide in 50 ml of toluene is added drop by drop
thereto within 90 minutes and the mixture is kept boiling for four more hours
while stirring. The cooled reaction mixture is allowed to run into 1.5 liters of
ice water and the mixture is acidified with 20% hydrochloric acid. The aqueous layer is separated and is rendered alkaline by the addition of a
solution of potassium carbonate. The base is taken up in warm benzene. The
solvent is evaporated and the residue is distilled in a vacuum. 62.6 g of α-
isopropyl-α-[(N-methyl-N-homoveratryl)-γ-aminopropy]-3,4-dimethoxyphenyl
acetonitrile are obtained in the form of a light yellow, very viscous oil. Boiling
point: 232°C to 235°C/0.01 mm Hg; n D 25 = 1.5460. Yield: 91.8% of the
theoretical yield. Hydrochloride: melting point: 139.5°C to 140.5°C (corr.), on
recrystallization from a mixture of isopropanol and ethyl acetate. | | Therapeutic Function | Coronary vasodilator, Antiarrhythmic | | General Description | Verapamil, 5-[. Hemodynamically, verapamil causesa change in the preload, afterload, contractility, heart rate,and coronary blood flow. The drug reduces systemic vascularresistance and mean blood pressure, with minor effectson cardiac output.
Verapamil is a synthetic compound possessing slightstructural similarity to papaverine. It can be separated intoits optically active isomers, of which the levorotatory enantiomeris the most potent. It is absorbed rapidly after oraladministration. The drug is metabolized quickly and, as aresult, has low bioavailability. The liver is the main siteof first-pass metabolism, forming several products. Thepreferential metabolic step involves N-dealkylation, followedby O-demethylation, and subsequent conjugation ofthe product before elimination. The metabolites have no significantbiological activity. Verapamil has an eliminationhalf-life of approximately 5 hours. | | Mechanism of action | Verapamil is used as an antiarrythmic drug in treating supraventricular arrythmia such as
paroxysmal atrial tachycardia, and for controlling atrial fibrillation. By blocking entrance
of Ca2+ in the cell, verapamil exhibits a negative inotropic effect, and therefore it cannot
be combined with β-adrenoblockers or cynidine since that would lead to an increased
inotropic effect. | | Clinical Use | Verapamil (Isoptin, Covera), in addition to its use as an
antiarrhythmic agent, has been employed extensively in
the management of variant (Prinzmetal’s) angina and
effort-induced angina pectoris. It selectively inhibits the voltage-gated calcium
channel that is vital for action potential genesis in slowresponse
myocytes, such as those found in the sinoatrial
and A-V nodes.
Verapamil is useful for slowing the ventricular response
to atrial tachyarrhythmias, such as atrial flutter and fibrillation.
Verapamil is also effective in arrhythmias supported
by enhanced automaticity, such as ectopic atrial
tachycardia and idiopathic left ventricular tachycardia. | | Side effects | Orally administered verapamil is well tolerated by most
patients. Most complaints are of constipation and gastric
discomfort. Other complaints include vertigo,
headache, nervousness, and pruritus. | | Synthesis | Verapamil, 5-[(3,4-dimethoxyphenethyl)methylamino]-2-(3,4-dimethoxyphenyl)
isopropylvaleronitrile (19.3.15), is synthesized by a scheme using 3,4-
dimethoxyphenylacetonitrile as the initial substance. The synthesis of the final product
(19.3.15) is accomplished by alkylating 2-(3.4-dimethoxyphenyl)-3-methylbutyronitrile
(19.3.11) with N-[2-(3,4-dimethoxyphenyl)-ethyl]-N-3-(chloropropyl)-N-methylamine
(19.3.14). The initial 2-(3.4-dimethoxyphenyl)-3-methylbutyronitrile (19.3.11) is synthesized
by alkylating 3,4-dimethoxyphenylacetonitrile with isopropyl chloride in the
presence of sodium amide. The alkylating agent, N-[2-(3,4-dimethoxyphenyl)-ethyl]-N-3-
(chloropropyl)-N-methylamine (19.3.14), is also synthesized from 3,4-dimethoxyphenylacetonitrile
followed by reduction into 3,4-dimethoxyphenylethylamine (19.3.12), with
subsequent methylation into N-methyl-N-3,4-dimethoxyphenylethylamine (19.3.13).
Next, the resulting N-[2-(3,4-dimethoxyphenyl)-ethyl] -N-methylamine (19.3.12) is
alkylated by 1-chloro-3-bromopropane into the desired N-[2-(3,4-dimethoxyphenyl)-
ethyl]-N-3-(chloropropyl)-N-methylamine (19.3.14), which is alkylated by 2-(3.4-
dimethoxyphenyl)-3-methylbutyronitrile (19.3.11) to give the final product, verapamil(19.3.15). 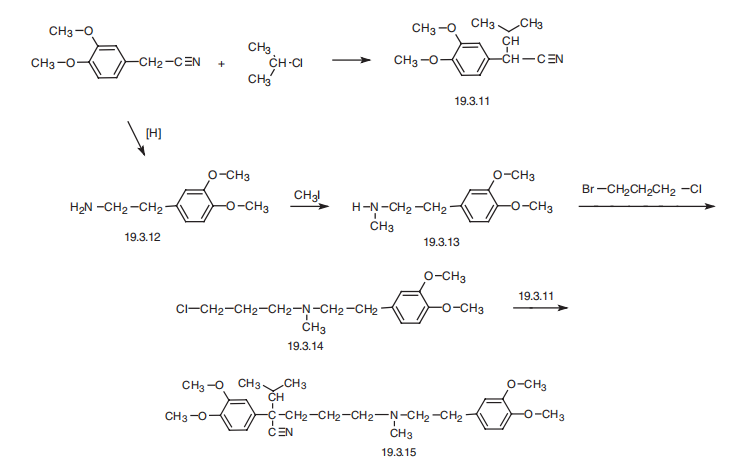
| | Precautions | Verapamil must be used with extreme caution or not at
all in patients who are receiving -adrenoceptor blocking
agents. Normally, the negative chronotropic effect of
verapamil will in part be overcome by an increase in reflex
sympathetic tone. The latter is be prevented by simultaneous
administration of a β-adrenoceptor blocking
agent, which exaggerates the depressant effects of verapamil on heart rate, A-V node conduction, and
myocardial contractility. The use of verapamil in children
less than 1 year of age is controversial. |
| | Verapamil Preparation Products And Raw materials |
|



