|
| | Ciclesonide Basic information |
| Product Name: | Ciclesonide | | Synonyms: | (r)-11b,16a,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with cyclohexanecarboxaldehyde 21-isobutyrate;Ciclesonide;(11β,16α)-16,17-[[(R)-CyclohexylMethylene]bis(oxy)]-11-hydroxy-21-(2-Methyl-1-oxopropoxy)-pregna-1,4-diene-3,20-dione;Ciclesonide(RPR251526);Ciclesonide containing impurity A CRS;Ciclesonide CRS;Zetonna;Pregna-1,4-diene-3,20-dione, 16,17-[[(R)-cyclohexylmethylene]bis(oxy)]-11-hydroxy-21-(2-methyl-1-oxopropoxy)-, (11β,16α)- | | CAS: | 126544-47-6 | | MF: | C32H44O7 | | MW: | 540.69 | | EINECS: | | | Product Categories: | Other APIs;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Steroids | | Mol File: | 126544-47-6.mol |  |
| | Ciclesonide Chemical Properties |
| Melting point | 202-209?C | | Boiling point | 665.0±55.0 °C(Predicted) | | density | 1.23±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,2-8°C | | solubility | Chlorofrom (Slightly), Methanol (Slightly) | | form | neat | | pka | 14.25±0.70(Predicted) | | color | White to Off-White | | InChIKey | LUKZNWIVRBCLON-GXOBDPJESA-N |
| | Ciclesonide Usage And Synthesis |
| Description | Ciclesonide, a new inhaled corticosteroid (ICS), is indicated for the prophylactic
treatment of persistent asthma. ICS treatment is a widely accepted standard of care
for maintenance therapy of chronic asthma, and the currently available agents include
fluticasone propionate, budesonide, triamcinolone acetonide, flunisolide, and
beclomethasone dipropionate. These agents exert their potent anti-inflammatory
effects via modulation of the glucocorticoid receptor (GR). Although ICS drugs are
generally safe and well tolerated compared with oral corticosteroids, many have
measurable systemic exposures, and concerns over potential side effects resulting
from it severely limit the dose at which they can be administered for long-term
therapy. Systemic adverse effects associated with corticosteroids include HPA axis
suppression, osteoporosis, abnormal glucose metabolism, cataracts, and glaucoma,
some of which could potentially occur with the long-term use of high dose ICS. The
key differentiators for ciclesonide relative to other ICS drugs are its longer duration
of action and lower systemic exposure. Ciclesonide is an isobutyryl ester prodrug. It is
cleaved by the endogenous esterases in the lung to des-isobutyryl ciclesonide (des-
CIC), which is a potent GR agonist. The binding affinity of des-CIC for human GR
(Ki=0.31 nM) is similar to other ICS such as budesonide (Ki=0.44nM) and fluticasone
propionate (Ki=0.24nM), while ciclesonide itself has about 100-fold lower
affinity (Ki=37nM). In lung tissue, des-CIC undergoes reversible lipid conjugation
to form oleate and palmitate ester conjugates, which act as a slow-release pool for the
drug and increase the pulmonary residence time. This, in turn, contributes to the
enhanced local effects and the long duration of action.
Inhaled ciclesonide was
generally well tolerated in these clinical studies. Ciclesonide did not suppress biochemical
markers of adrenal function in 52-week studies; however, the long-term
(>52 weeks) systemic effects remain unknown. Ciclesonide is chemically produced
via a semi-synthesis starting from 16-α-hydroxyprednisolone by first converting to a
triisobutyryl ester intermediate with isobutyric anhydride, and subsequent reaction of
the triester with cyclohexane carboxaldehyde and hydrochloric acid in dioxane. The
latter step produces the cyclic ketal as a mixture of diastereomers, which is subjected
to HPLC and fractional crystallization to produce ciclesonide. | | Chemical Properties | White Solid | | Originator | Recordati
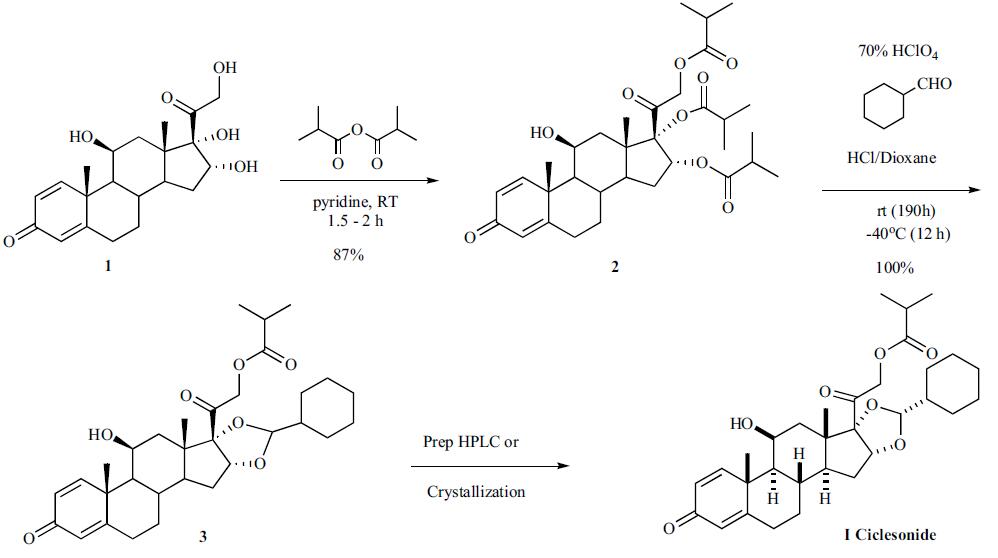
Espana (Spain) | | Uses | A glucocorticoid microemulsion nasal preparation allergy inhibitor rhinitis. | | Definition | ChEBI: Ciclesonide is an organic molecular entity. | | Brand name | Alvesco (Dynamit Nobel GmbH). | | General Description | Ciclesonide (Omnaris) is a prodrug that requireshydrolysis of the isobutyrate ester at C21 to form theactive corticosteroid (des-ciclesonide). It has minimal oralbioavailability due to extensive metabolism, mainly byCYP3A4. The metabolites of ciclesonide have not beenfully characterized. | | Synthesis | Two separate approaches to the
syntheses of the chiral ciclesonide have been described in the
patent literature. The first route involves a chiral resolution
step and the second approach highlights a stereoselective
trans acetalization approach. The first synthesis of
ciclesonide started by reacting (11|?,16|á)-11,
16,17,21-tetrahydroxypregna-1,4-diene-3,20-dione (1) with
isobutyric anhydride to make the tri-isobutyl ester in 87%
yield. Reaction of the tri-ester with cyclohexane carboxaldehyde
in the presence of HCl and 70% perchloric acid gave
the cyclohexane acetal 3, which was then separated into the
desired isomer ciclesonide (I) by HPLC or recrystallization.
| | storage | Store at +4°C |
| | Ciclesonide Preparation Products And Raw materials |
|