|
| | Indapamide Chemical Properties |
| WGK Germany | 2 | | RTECS | CV2451200 | | HS Code | 2935904000 | | Toxicity | LD50 in rats, mice, guinea pigs (mg/kg): 393-421, 410-564, 347-416 i.p.; 394-440, 577-635, 272-358 i.v.; >3000 all species orally (Kyncl) |
| | Indapamide Usage And Synthesis |
| Diuretic antihypertensive drug | White needle crystal or crystalline powder, odorless, tasteless. It is almost insoluble in water or dilute hydrochloric acid, while it can be dissolved in ethanol or ethyl acetate, and it is soluble in acetone, acetic acid, slightly soluble in chloroform or ether.
Indapamide is currently the most popular non-prescription diuretic antihypertensive drug with good efficacy, stable blood pressure, fewer side effects, etc. It was originally developed for the first time by the French Servier (Servier) pharmaceutical company. Indapamide film-coated tablets were first successfully developed by Tianjin Lisheng pharmaceutical company in 1988 in China. The trade name is "life than the mountains." In the mid-1990s, Zhejiang Apeloa pharmaceutical, Yantai Xiyuan pharmaceutical factory, Zhejiang East medicine, Dongguan million into pharmaceuticals, Shanxi Asia-medicine, medicine Fuxin Shibata, Puyang the yuan Pharmaceutical, Chongqing Friends of pharmaceutical drugs, 8 pharmaceutical formulations were approved for production. In the late 1990s, the French pharmaceutical company Servier took indapamide sustained-release tablets into China. The trade name is "Na Ionizers." Subsequently, indapamide raw material drug localization has been progress. Currently, seven companies have been allowed to produce raw material drug types.
Indapamide have diuretic and calcium antagonist dual effect by inhibiting the proximal end of the distal convoluted tubule Na+ reabsorption, resulting in diuresis, while by blocking Ca2+ influx especially a higher selectivity for vascular smooth muscle to dilate the small blood vessels of the outer periphery, resulting in antihypertensive effect. But the effect to vascular smooth muscle is stronger than the diuretic effect. It can lower blood pressure with lower dose compared to diuretic effect. Higher dose will display diuretic effect. But there is no disadvantage compared to thiazide diuretics, that it does not cause orthostatic hypotension, flushing and reflex tachycardia, nor blood lipids, glucose metabolism and renal function. The therapeutic dosage for heart rate, cardiac output, electrocardiogram are no significant change, as well as for the central nervous system and autonomic. There is antihypertensive effect by oral for 2~3h, maintaining 24h. single medication has good effect. Diuretic effect appears at 3h, achieving maximum effect for 4~6h. It is different from other diuretics. This product is fat-soluble. After oral administration, there is highest concentration in the liver, renal plasma, and lower concentration in heart, lung, muscle, fat. This product excretes from the kidney mainly by metabolites and 5% of the prototype. Indapamide is for mild to moderate hypertension, and for sodium retention caused by congestive heart failure. It is also applied to hypertension with renal failure, diabetes mellitus, high blood lipids. Single medication has significant effect. It is combined with β-receptor blockers that has better effect. Because the drug has a diuretic effect, it can cause hypokalemia, which can add potassium.
The above information is edited by the chemicalbook of Kui Ming.
| | Uses | For the treatment of mild to moderate essential hypertension.
| | Category toxic | Substances
| | Toxicity grading | Medium toxicity | | Acute toxicity | Oral-rat LD50:> 3000 mg/kg; Oral-mouse LD50:> 3000 mg/kg.
| | Flammability hazard characteristics | Combustible; combustion produces toxic nitrogen oxides, sulfur oxides and chlorides smoke. | | Storage characteristics | Treasury ventilation low-temperature drying.
| | Extinguishing agent | Dry powder, foam, sand, carbon dioxide, water mist. | | Description | Indapamide is a derivative of benzolsulfonamide and its mechanism of action is analogous to
that of thiazides. It is intended for lowering arterial blood pressure and as an adjuvant drug for
treating edema caused by cardiac insufficiency. | | Description | Indapamide (Item No. 21308) is an analytical reference standard that is categorized as a sulfonamide diuretic that blocks delayed-rectifier potassium currents. Formulations containing indapamide are used to treat hypertension, but it is abused by athletes to reduce body weight rapidly or mask the presence of other athletic-enhancing substances. Indapamide can be detected in urine. This product is intended for research and forensic applications. | | Chemical Properties | Crystalline Solid | | Originator | Natrilix,Pharmacodex,W. Germany,1976 | | Uses | Used as an antihypertensive. Diuretic. | | Uses | diuretic, antihypertensive | | Definition | ChEBI: A sulfonamide formed by condensation of the carboxylic group of 4-chloro-3-sulfamoylbenzoic acid with the amino group of 2-methyl-2,3-dihydro-1H-indol-1-amine. | | Manufacturing Process | A total of 8.9 parts of 3-sulfamyl-4-chloro-benzoylchloride in a solution of 50
parts of anhydrous tetrahydrofuran are added portionwise in the course of 60
minutes, while stirring, to a solution of 5.2 parts of N-amino-2-methyl indoline
and 3.5 parts of triethylamine in 150 parts of anhydrous tetrahydrofuran. The
reaction mixture is left to stand 3 hours at room temperature, then the
precipitated chiorhydrate of triethylamine is filtered off. The filtrate is
evaporated under vacuum and the residue is crystallized from a solution of 60
parts of isopropanol in 75 parts of water. There are obtained 9 parts of N-(3-
sulfamyl-4-chlorobenzamido)-2-methyl indoline, MP (K) 184° to 186°C, MP
(MK) 160° to 162°C (isopropanol/water). [The melting points beingdetermined on a Kofler heater plate under the microscope (MK) or on a Kofler
Bank (K)]. | | Brand name | Lozol
(Sanofi Aventis). | | Therapeutic Function | Diuretic Indapamide | | Clinical Use | Indapamide is an effective
diuretic drug when GFR falls below 40 mL/min. The duration of action is approximately 24 hours, with the normal oral adult dosage starting at 2.5 mg given each morning.
The dose may be increased to 5.0 mg/day, but doses beyond this level do not appear to provide additional results. | | Side effects | Effects on urine content and side effects are similar to
effects induced by thiazide diuretics. | | Synthesis | Indapamide, 4-chloro-N-(2-methyl-1-indolinyl)-3-sulfamoylbenzamide
(21.3.33), is synthesized from 2-methylendoline, the nitrosation of which gives 2-methyl-
1-nitrosoindoline (21.3.31). Reducing this with lithium aluminum hydride leads to formation
of 1-amino-2-methylendoline (21.3.32). Acylating this with 3-sulfonylamino-4-chlorbenzoic
acid chloride leads to (21.3.33). 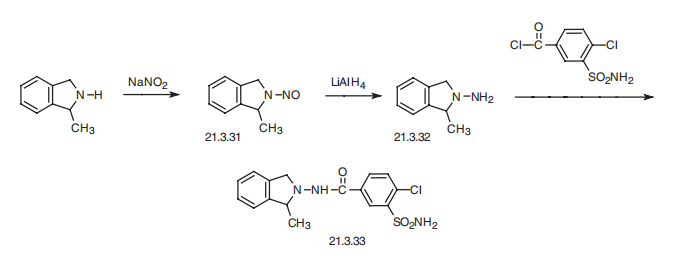
| | Drug interactions | Potentially hazardous interactions with other drugs
Analgesics: increased risk of nephrotoxicity with
NSAIDs; antagonism of diuretic effect.
Anti-arrhythmics: hypokalaemia leads to increased
cardiac toxicity; effects of lidocaine and mexiletine
antagonised.
Antibacterials: avoid administration with
lymecycline.
Antidepressants: increased risk of hypokalaemia
with reboxetine; enhanced hypotensive effect with
MAOIs; increased risk of postural hypotension with
tricyclics.
Antiepileptics: increased risk of hyponatraemia with
carbamazepine.
Antifungals: increased risk of hypokalaemia with
amphotericin.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotension with postsynaptic
alpha-blockers like prazosin; hypokalaemia
increases risk of ventricular arrhythmias with sotalol.
Antipsychotics: hypokalaemia increases risk
of ventricular arrhythmias with amisulpride;
enhanced hypotensive effect with phenothiazines;
hypokalaemia increases risk of ventricular
arrhythmias with pimozide - avoid.
Atomoxetine: hypokalaemia increases risk of
ventricular arrhythmias.
Cardiac glycosides: increased toxicity if hypokalaemia
occurs.
Ciclosporin: increased risk of nephrotoxicity and
possibly hypomagnesaemia.
Cytotoxics: increased risk of ventricular arrhythmias
due to hypokalaemia with arsenic trioxide; increased
risk of nephrotoxicity and ototoxicity with platinum
compounds.
Lithium excretion reduced (increased toxicity). | | Metabolism | Indapamide is strongly bound to red blood cells, and
is taken up by the vascular wall in smooth vascular
muscle according to its high lipid solubility. 60-70% of
a single oral dose is eliminated by the kidneys and 23%
by the gastrointestinal tract. Indapamide is extensively
metabolised with 5-7% of unchanged drug found in
the urine during the 48 hours following administration.
About 16-23% of dose is excreted in the faeces |
| | Indapamide Preparation Products And Raw materials |
|



