| Chemical Properties | Alginic acid is a tasteless, practically odorless, white to yellowishwhite,
fibrous powder. |
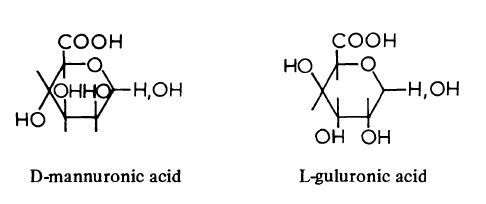
| Chemical Properties | Alginic acid found in the walls of brown seaweeds (Phaeophyceae) consists of D-mannuronic acid units and L-guluronic acid units probably linked β (1->4). The chains contain three distinct regions; in one, the D-mannuronic acid units alternate with L-guluronic acid units, whereas the remaining regions are homogeneous and contain either D-mannuronic acid or L-guluronic acid. The lengths and proportions of the three regions vary with the seaweed species and are responsible for the differences in chemical and physical properties of the various alginates.
Alginic acid is biosynthesised from GDP-D-mannuronic acid and GDP-guluronic acid. It seems probable that guluronic acid is derived from its isomer, mannuronic acid, the re action being catalysed by an epimerase.
Monovalent salts of alginic acid are soluble in water, but polyvalent salts are either insoluble or form gels. Solutions of alginates are very viscous due to the high molecular weight and random-coil formation of the polymers. The jellying and thickening properties of alginates are widely used commercially in foodstuffs, etc.
|
| Uses | Gum derived from alginic acid which is obtained from brown seaweed genera, such as Macrocystis pyrifera. The derivatives are sodium, ammonium, and potassium ates of which the sodium salt is most common. They are used to provide thickening, gelling, and binding. A derivative designed for improved acid and calcium stability is propylene glycol ate. The s are soluble in cold water and form nonthermoreversible gels in reaction with calcium ions and under acidic conditions. Algin is used in ice cream, icings, puddings, dessert gels, and fabricated fruit. |
| Uses | corrosive moisture sensitive |
| Uses | algin (alginic acid) is used in cosmetic formulations as a thickener, stabilizer, and gelling agent. It is obtained from different varieties of brown seaweed. |
| Uses | Alginic Acid is the acidic, insoluble form of algin that is a white to
yellowish fibrous powder obtained from brown seaweed genera,
such as macrocystis pyrifera. the derivatives are soluble and include
sodium, potassium, and ammonium alginate and propylene glycol
alginate. it is used as a tablet disintegrant and as an antacid
ingredient. |
| Definition | A polysaccharide composed of β,d-mannuronic acid residues linked so that the carboxyl group of each unit is free, while the aldehyde group is shielded by a glycosidic linkage. It is a linear polymer of the mannuronic acid in the pyranose ring form. |
| Production Methods | Alginic acid is a hydrophilic colloid carbohydrate that occurs
naturally in the cell walls and intercellular spaces of various species
of brown seaweed (Phaeophyceae). The seaweed occurs widely
throughout the world and is harvested, crushed, and treated with
dilute alkali to extract the alginic acid. |
| Definition | A yellow-white organic solid
that is found in brown algae. It is a complex
polysaccharide and produces, in even
very dilute solutions, a viscous liquid. Alginic
acid has various uses, especially in the
food industry as a stabilizer and texture
agent. |
| Pharmaceutical Applications | Alginic acid is used in a variety of oral and topical pharmaceutical
formulations. In tablet and capsule formulations, alginic acid is used
as both a binder and disintegrating agent at concentrations of 1–5%
w/w. Alginic acid is widely used as a thickening and suspending
agent in a variety of pastes, creams, and gels; and as a stabilizing
agent for oil-in-water emulsions.
Alginic acid has been used to improve the stability of
levosimendan.
Therapeutically, alginic acid has been used as an antacid.In
combination with an H2-receptor antagonist, it has also been
utilized for the management of gastroesophageal reflux. |
| Safety Profile | Moderately toxic by intraperitoneal route. When heated to decomposition it emits acrid smoke and irritating fumes |
| Safety | Alginic acid is widely used in food products and topical and oral
pharmaceutical formulations. It is generally regarded as a nontoxic
and nonirritant material, although excessive oral consumption may
be harmful. Inhalation of alginate dust may be an irritant and has
been associated with industrially related asthma in workers
involved in alginate production. However, it appears that the cases
of asthma were linked to exposure to unprocessed seaweed dust
rather than pure alginate dust. An acceptable daily intake of
alginic acid and its ammonium, calcium, potassium, and sodium
salts was not set by the WHO because the quantities used, and the
background levels in food, did not represent a hazard to health.
LD50 (rat, IP): 1.6 g/kg |
| storage | Alginic acid hydrolyzes slowly at warm temperatures producing a
material with a lower molecular weight and lower dispersion
viscosity.
Alginic acid dispersions are susceptible to microbial spoilage on
storage, which may result in some depolymerization and hence a
decrease in viscosity. Dispersions should therefore be preserved with
an antimicrobial preservative such as benzoic acid; potassium
sorbate; sodium benzoate; sorbic acid; or paraben. Concentrations
of 0.1–0.2% are usually used.
Alginic acid dispersions may be sterilized by autoclaving or
filtration through a 0.22 μm filter. Autoclaving may result in a
decrease in viscosity which can vary depending upon the nature of
any other substances present.
Alginic acid should be stored in a well-closed container in a cool,
dry place. |
| Purification Methods | To 5g of acid in 550mL water containing 2.8g KHCO3 are added 0.3mL of acetic acid and 5g potassium acetate. EtOH is added to make the solution to 25% (v/v) in EtOH, and any insoluble material is discarded. Further addition of EtOH, to 37% (v/v), precipitated alginic acid. Collect the acid and dry it in vacuo. [Pal & Schubert J Am Chem Soc 84 4384 1962.] |
| Incompatibilities | Incompatible with strong oxidizing agents; alginic acid forms
insoluble salts in the presence of alkaline earth metals and group III
metals with the exception of magnesium. |
| Regulatory Status | GRAS listed. Accepted in Europe for use as a food additive.
Included in the FDA Inactive Ingredients Database (ophthalmic
preparations, oral capsules, and tablets). Included in the Canadian
List of Acceptable Non-medicinal Ingredients. Included in nonparenteral
medicines licensed in the UK. |


