|
| | 2,2,6,6-Tetramethylpiperidine Basic information |
| Product Name: | 2,2,6,6-Tetramethylpiperidine | | Synonyms: | HTMP;2,2,6,6-TETRAMETHYLPIPERIDINE;2,2,6,6-TetramethyL;2,2,6,6-Teteamethyl-piperidine;2,2,6,6-Tetramethylpiperidine(Tmp);2,2,6,6-tetramethyl-piperidin;Norpempidine;2,2,6,6-Tetramethylpiperidine (HTMP) | | CAS: | 768-66-1 | | MF: | C9H19N | | MW: | 141.25 | | EINECS: | 212-199-3 | | Product Categories: | Fine Chemical;Industrial/Fine Chemicals;Piperidines, Piperidones, Piperazines;Piperidine;API intermediates;Organic BasesBuilding Blocks;Chemical Synthesis;Heterocyclic Building Blocks;Piperidines;Synthetic Reagents;768-66-1 | | Mol File: | 768-66-1.mol |  |
| | 2,2,6,6-Tetramethylpiperidine Chemical Properties |
| Hazard Codes | Xn,F,C | | Risk Statements | 10-22-36/37/38 | | Safety Statements | 26-37/39-16 | | RIDADR | UN 1992 3/PG 3 | | WGK Germany | 2 | | RTECS | TN4220000 | | Autoignition Temperature | 290 °C | | TSCA | Yes | | HazardClass | 3 | | PackingGroup | III | | HS Code | 29333999 | | Toxicity | LD50 orally in Rabbit: 500 mg/kg |
| | 2,2,6,6-Tetramethylpiperidine Usage And Synthesis |
| Chemical Properties | clear colorless to light yellow-green liquid | | Uses | 2,2,6,6-Tetramethylpiperidine is used in the syntheses of HMP-Y1, Hibarimicinone and HMP-P1, tyrosine kinase inhibitors. | | Uses | 2,2,6,6-Tetramethylpiperidine is a hindered base used to prepare metallo-amide bases and selective generation of silylketene acetals. It is used in the preparation of hibarimicinone, (Z)-silylketene acetal and 4-substituted quinazoline. It acts as a precursor to Lithium tetramethylpiperidide and (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl radical. | | Uses | The lithium amide has been used to efficiently ortho deprotonate pyridine-3-carboxamides. | | Preparation |
Many routes for the synthesis of TMP have been reported. One method starts with a conjugate addition reaction of ammonia to phorone. The intermediate triacetone amine is then reduced in a Wolff-Kishner reaction.
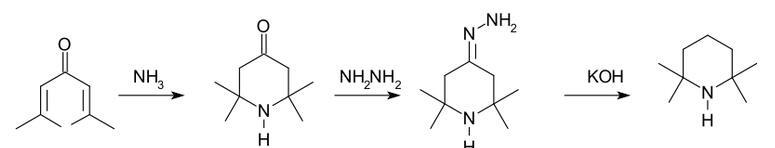
|
| | 2,2,6,6-Tetramethylpiperidine Preparation Products And Raw materials |
|



