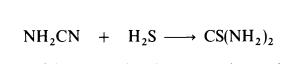| Description | Thiourea appears as white crystal/powder, is combustible, and on contact with fire, gives
off irritating or toxic fumes/gases. Thiourea is a reducing agent used primarily in the production
of bleached recycled pulp. In addition, it is also effective in the bleaching of stone
groundwood, pressurised groundwood. Thiourea undergoes decomposition on heating
and produces toxic fumes of nitrogen oxides and sulphur oxides. It reacts violently with
acrolein, strong acids, and strong oxidants. The main application of thiourea is in textile
processing and also is commonly employed as a source of sulphide. Thiourea is a precursor
to sulphide to produce metal sulphides, for example, mercury sulphide, upon reaction
with the metal salt in aqueous solution. The industrial uses of thiourea include production
of flame-retardant resins and vulcanisation accelerators. Thiourea is used as an auxiliary
agent in diazo paper, light-sensitive photocopy paper, and almost all other types
of copy paper. Thiourea is used in many industrial applications, including as a chemical
intermediate or catalyst, in metal processing and plating, and in photoprocessing. |
| Chemical Properties | Thiourea consists of colorless, lustrous crystals or powder with a bitter taste. |
| Chemical Properties | white crystals or powder |
| Uses | The product is wildly used in pharmaceutical industry, agricultural, chemicals, metallurgical industry, petroleum and so on. It is also main material for producing thiourea dioxide(CH1N2O2S).
|
| Uses | Chaotropic agent; strong denaturant. Increases solubility and recovery of proteins |
| Uses | Used in determination of bismuth. |
| Uses | In animal glue liquifiers and silver tarnish removers. Photographic fixing agent and to remove stains from negatives; manufacture of resins; vulcanization accelerator; a reagent for bismuth, selenite ions. |
| Uses | Thiourea is used in the manufacture of resins,as a vulcanization accelerator, and as aphotographic fixing agent and to removestains from negatives. |
| Uses | The most common uses for thiourea have been for the production of thiourea dioxide (30%), in leaching of gold and silver ores (25%), in diazo papers (15%), and as a catalyst in the synthesis of fumaric acid (10%) (IARC 2001). It has also been used in the production and modification of synthetic resins. Other uses of thiourea are as a photographic toning agent, in hair preparations, as a drycleaning agent, in the synthesis of pharmaceuticals and pesticides, in boiler-water treatment, and as a reagent for bismuth and selenite ions. It has also been used in textile and dyeing auxiliaries, in the production of industrial cleaning agents (e.g., for photographic tanks and metal surfaces in general), for engraving metal surfaces, as an isomerization catalyst in the conversion of maleic to fumaric acid, in copper-refining electrolysis, in electroplating, and as an antioxidant. Other uses have included as a vulcanization accelerator, an additive for slurry explosives,as a viscosity stabilizer for polymer solutions, and as a mobility buffer in petroleum extraction. It is also used as an ingredient of consumer silver polishes (HPD 2009), and has been used in the removal of mercury from wastewater by chlorine-alkali electrolysis (IARC 1974, 2001, WHO 2003). |
| Production Methods | Thiourea is formed by heating ammonium thiocyanate at 170 °C (338 °F). After about an hour, 25% conversion is achieved. With HCl, thiourea forms thiourea hydrochloride; with mercuric oxide, thiourea forms a salt; and with silver chloride, it forms a complex salt. |
| Preparation | Thiourea is manufactured by heating ammonium thiocyanate at 140-145??C
for about 4 hours; equilibrium is established when about 25% of the
thiocyanate is converted to thiourea.Thiourea may also be prepared by the interaction of cyanamide and hydrogen sulphide:
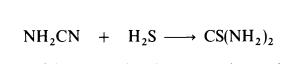
Thiourea closely resembles urea in that reaction with formaldehyde gives
methylol derivatives and then resinous condensates which on continued
heating yield network structures. Thiourea-formaldehyde resins are slower
curing than urea-formaldehyde resins and the hardened products are more
brittle and more water-resistant. At one time thiourea-formaldehyde resins
were added to urea-formaldehyde resins to give mouldings and laminates
with improved water-resistance. These mixed resins have now been largely
superseded by melamine-formaldehyde resins which give products with better
resistance to heat. |
| Definition | A colorless
crystalline organic compound (the sulfur
analog of urea). It is converted to the inorganic compound ammonium thiocyanate
on heating. It is used as a sensitizer in photography and in medicine. |
| Definition | thiourea: A white crystalline solid,(NH2)2CS; r.d. 1.4; m.p. 182°C. It isused as a fixer in photography. |
| Definition | ChEBI: The simplest member of the thiourea class, consisting of urea with the oxygen atom substituted by sulfur. |
| General Description | White or off-white crystals or powder. Sinks and mixes with water. |
| Air & Water Reactions | Water soluble. |
| Reactivity Profile | Thiocarbamide is a white crystalline material or powder, toxic, carcinogenic. When heated to decomposition Thiocarbamide emits very toxic fumes of oxides of sulfur and oxides of nitrogen. Violent exothermic polymerization reaction with acrylaldehyde (acrolein) [MCA SD-85, 1961], violent decomposition of the reaction product with hydrogen peroxide and nitric acid [Bjorklund G. H. et al., Trans. R. Soc. Can.,1950, 44, p. 28], spontaneous explosion upon grinding with potassium chlorate [Soothill, D., Safety Management, 1992, 8(6), p. 11]. |
| Hazard | A questionable carcinogen. May not be
used in food products (FDA); skin irritant (allergenic). |
| Health Hazard | Poisonous inhaled or swallowed. Irritating to skin; may cause allergic skin eruptions. |
| Health Hazard | The acute oral toxicity of thiourea in mostanimals is of low order. The oral LD50 values reported in the literature show variation.Symptoms of chronic effects in rats includebone marrow depression and goiters. Administration of 32.8 mol of thiourea in chickembryos on day 17 of incubation resultedin the accumulation of parabronchial liquidin those embryos (Wittman et al. 1987). Theinvestigators have attributed such changes tothe toxic effects of thiourea, rather to than aretardation of pulmonary development.Dedon and coworkers (1986) observed thepossible protective action of thiourea againstplatinum toxicity. Thiourea and other sulfur-containing nucleophiles have the ability tochelate and remove platinum from biochemical sites of toxicity.Oral administration of thiourea resultedin tumors in the liver and thyroid in rats.It is carcinogenic to animals and has shownsufficient evidence. |
| Fire Hazard | Noncombustible solid. There is no report of
any explosion resulting from reactions of
thiourea. Small amounts of thiourea in contact with acrolein may polymerize acrolein,
which is a highly exothermic reaction. |
| Agricultural Uses | Thiourea is a sulphur analogue of urea. It is a crystalline
and colorless solid which is relatively insoluble in water.
Thiourea, capable of breaking the dormancy of seeds, is
used to stimulate seed germination. Seeds are soaked for
less than 24 hours before planting. |
| Contact allergens | Thiourea is used as a cleaner agent for silver and cop-
per, and as an antioxidant in diazo copy paper. It can
induce (photo-) contact dermatitis. |
| Biochem/physiol Actions | Thiourea is a free radical scavenger of the peroxide radical. It was shown to inhibit lipid peroxidation and ultraviolet (UV)-induced crosslinking of collagen. Bud dormancy in plants can be inhibited by thiourea, which is used as a growth stimulator. It is also known to be used in the treatment of hyperthyroidism. |
| Potential Exposure | Thiourea is used as rubber antiozonant, toning agent; corrosion inhibitor; and in pharmaceutical manufacture; in the manufacture of photosensitive papers; flame-retardant textile sizes; boiler water treatment. It is also used in photography; pesticide manufacture; in textile chemicals. |
| Carcinogenicity | Thiourea is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental
animals. |
| Shipping | UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. |
| Purification Methods | Crystallise thiourea from absolute EtOH, MeOH, acetonitrile or water. Dry it under vacuum over H2SO4 at room temperature. [Beilstein 3 IV 342.] |
| Incompatibilities | Dust may form explosive mixture with air. Reacts violently with acrolein, strong acids (nitric acid). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. |
| Waste Disposal | Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. |