| Description | As a derivative of phenothiazine, promethazine is structurally and pharmacologically similar
to chlorpromazine. It exhibits strong antihistamine activity as well as expressed action
on the CNS. It potentiates action of sedative and analgesic drugs. |
| Uses | Anti-emetic; antihistaminic. |
| Uses | Promethazine is used in preparation of antibodies having inhibitory activities to IL-36R signaling triggered by agonistic ligands and application to prevention and therapies of cancers and autoimmune diseases. |
| Uses | Promethazine is used for treating allergic illnesses such as hives, serum disease, hay
fever, dermatosis, and also for rheumatism with expressed allergic components, for allergic
complications caused by antibiotics and other medicinal drugs, and for enhancing
action of analgesics and local anesthetics. Synonyms of this drug are allergen, phenergan,
pipolphen, prothazine, and others. |
| Definition | ChEBI: A tertiary amine that is a substituted phenothiazine in which the ring nitrogen at position 10 is attached to C-3 of an N,N-dimethylpropan-2-amine moiety. |
| Brand name | Phenergan (Wyeth). |
| World Health Organization (WHO) | Introduced in 1946, promethazine, a phenothiazine derivative has
a variety of pharmacological properties. At present it is mainly used as an
antihistamine and anti-motion-sickness drug. Promethazine is listed in the WHO
Model List of Essential Drugs. |
| General Description | Crystals. Melting point 60°C. Used as an antihistaminic. |
| Air & Water Reactions | Turns blue on prolonged exposure to air and moisture. |
| Reactivity Profile | PROMETHAZINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable or toxic gases may be generated in combination with strong reducing agents, such as hydrides. |
| Health Hazard | SYMPTOMS: Symptoms of PROMETHAZINE include leucopenia; agranulocytosis; confusion; convulsions; stupor; and it potentiates the action of central nervous system depressants. |
| Fire Hazard | Flash point data for PROMETHAZINE are not available, however PROMETHAZINE is probably combustible. |
| Clinical Use | Promethazine, an early agent in the series, has many useful pharmacological affects other
than being an antihistamine. It has significant antiemetic and anticholinergic properties. It
also has sedative-hypnotic properties and has been used to potentiate the effects of
analgesic drugs. Subsequent analogues, such as trimeprazine and methdilazine, are used as
antipruritic agents in the treatment of urticaria. |
| Safety Profile | Poison by ingestion,
intravenous, intramuscular, intraperitoneal,
and subcutaneous routes. Human systemic
effects by ingestion: pupillary dilation,
wakefulness, hallucinations, and distorted
perceptions. An experimental teratogen.
Other experimental reproductive effectsHuman mutation data reported. A severe
eye irritant. When heated to decomposition
it emits very toxic fumes of NOx and SOx |
| Synthesis | Promethazine, 10-(2-dimethylaminopropyl)phenothiazine (16.1.18), is
synthesized by alkylating phenothiazine with 1-dimethylamino-2-propylchloride. 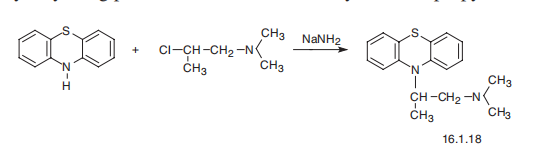
|



