|
| | Triethyl phosphite Basic information |
| | Triethyl phosphite Chemical Properties |
| Melting point | -112°C | | Boiling point | 65 °C | | density | 0.969 g/mL at 25 °C(lit.) | | vapor pressure | <6 hPa (20 °C) | | refractive index | n20/D 1.413(lit.) | | Fp | 130 °F | | storage temp. | Flammables area | | solubility | Chloroform (Slightly), Dichloromethane (Slightly), Methanol (Slightly) | | form | liquid | | color | colorless | | Specific Gravity | 0.969 | | Odor | characteristic, obnoxious, phosphiteodor | | explosive limit | 3.75-42.5%(V) | | Water Solubility | slightly soluble | | Sensitive | Air & Moisture Sensitive | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | BRN | 956578 | | Stability: | Air Sensitive, Moisture Sensitive | | CAS DataBase Reference | 122-52-1(CAS DataBase Reference) | | NIST Chemistry Reference | Phosphorous acid, triethyl ester(122-52-1) | | EPA Substance Registry System | Triethyl phosphite (122-52-1) |
| | Triethyl phosphite Usage And Synthesis |
| Chemical Properties | Triethyl phosphite is a clear colorless liquid with a strong foul odor. Insoluble in water; soluble in alcohol and ether. Combustible.Vapors heavier than air. | | Uses | Synthesis, plasticizers, stabilizers, lubricant and
grease additives. | | Uses | Triethyl phosphite is an organophosphorus compound. It is used as a reducing agent; can react with electrophiles to form phosphonates or phosphates; forms a stable complex with copper(I) iodide.
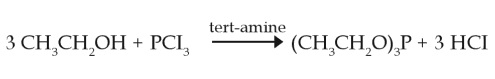
Triethyl phosphite is a very good nucleophile. The carbon adjacent to the bromine is the most electrophilic position, and phosphorus is the only nucleophile. Triethyl phosphite displaces the bromine in an SN2-like process, and back attack by the bromide which is released generates a phosphonate product, in which the α-protons are strongly acidic. | | Uses | Triethyl phosphite is used as a ligand in organometallic chemistry and as a reagent in organic synthesis. It is used as reference to phosphoric acid standard in 31P NMR spectroscopy. It acts as a reducing agent to prepare 2-phenylindazole from o-nitrobenzylidineaniline as well as reacts with electrophiles to get phosphonates. It forms a stable complex with copper(I) iodide. It finds application in a modified Staudinger reaction for the alkyl bromide to an amine through the azide. Further, it is also used in the preparation of 3-phenyl-2-substituted indoles by reacting with beta-nitro styrenes. | | Preparation | Triethyl phosphite is produced in a closed system by reaction of phosphorous trichlorid and ethanol in the presence of an inorganic or organic base. The product is purified by distillation (Bayer AG, 2002a).
Triethyl phosphite is exclusively used as an intermediate for the manufacturing of different products: flame retardants (about 60 %), optical brighteners (about 15 %), pesticides (about 15 %), antioxidants (about 5 %), and pharmaceuticals (about 5 %). | | Reactions | This reaction is currently used for the preparation of synthetically useful phosphonate reagents employed in modified retinal studies. Thus, diethyl 3-alkoxycarbonyl-2-propenylphosphonates are prepared in 72-91% yields by the reaction of methyl or ethyl 4-bromocrotonates with triethyl phosphite at 150-160°C. Similarly, diethyl 3-(ethoxycarbonyl)-2 methyl-2-propenylphosphonate is prepared in 81% yield from triethyl phosphite and ethyl 3-methyl-4-chlorocrotonate by heating at 180- 200°C.The diethyl (E)- and (Z)-3-ethoxycarbonyl-3 fluoro-2 -methyl-2-propenylphosphonates are respectively obtained from triethyl phosphite and (E)- or (Z)-4-bromo-2-fuoro-3-methyl-2-butenoates at 140°C. | | Reactivity Profile | Triethyl phosphite is colorless, moderately toxic liquid, combustible. Flammable when exposed to heat or flame. When heated to decomposition Triethyl phosphite emits toxic fumes of oxides of phosphorus [Lewis, 3rd ed., 1993, p. 1271]. | | Health Hazard | Exposure to high concentrations may cause headache, nausea, and dizziness due to reduced chlolinesterase activity. | | Fire Hazard | Special Hazards of Combustion Products: May form hazardous decomposition products. | | Flammability and Explosibility | Flammable | | Safety Profile | Moderately toxic by
ingestion. A skin and eye irritant. Flammable
liquid when exposed to heat, sparks, or
flame. When heated to decomposition it
emits toxic fumes of POx. | | Toxicity evaluation | The acute toxicity after oral, dermal, and inhalation exposure is relatively low. The oral LD50s in rats ranged between 1840 mg/kg bw (females) and 2470 mg/kg bw (males). Symptoms of rapid breathing and tremors were observed prior to death. In mice LD50 values above 3700 mg/kg bw were recorded. The 6-hour inhalation LC50 with an aerosol of 1.6-3.5 μm MMAD in rats was between 11,100 mg/m3 (females) and 11,600 mg/m3 (males). Clinical signs included eye and upper respiratory irritation, salivation and rapid, shallow breathing. The dermal LD50 in rabbits was between 2800 mg/kg bw (males) and > 3000 mg/kg bw (females). |
| | Triethyl phosphite Preparation Products And Raw materials |
| Raw materials | Ammonium chloride-->Xylene-->Phosphorus trichloride-->Diethyl phosphite-->Methyl Red | | Preparation Products | 4,4'-Vinylenedipyridine-->Cyclopentane-1,1-diacetic acid-->SPIRO[4.5]DECANE-7,9-DIONE-->(2-Aminoethyl)phosphonic acid-->FONOFOS-->1-CYCLOPENTYLIDENE-PROPAN-2-ONE-->2-(2,3-DIHYDRO-1BENZENESULFONYL-PYRROLO[2,3-B]PYRIDIN-3-YL)ETHANAMINE-->2-(2,3-DIHYDRO-1-BENZENESULFONYL-PYRROLO[2,3-B]PYRIDIN-3-YL)ACETONITRILE-->4-Benzofurazancarboxaldehyde-->2-METHYLCYCLOPROPANECARBOXYLIC ACID-->Foscarnet sodium-->THIOPHEN-2-YLMETHYL-PHOSPHONIC ACID DIETHYL ESTER-->GERANIC ACID-->7-METHYL-1,6-OCTADIENE-->Propylphosphonic anhydride-->4-Oxo-9,10-dihydro-4H-benzo(4,5)-cyclohepta-(1,2b)thiophene-->DIETHYL (N-METHOXY-N-METHYLCARBAMOYLMETHYL)PHOSPHONATE-->N-(2-Methylpropylidene)-butylamine-->2-(b-(2-Thienyl)vinyl)benzoicacid-->2-[2-(2-thienyl)ethyl]benzoic acid-->DIETHYL(4-NITROBENZYL)PHOSPHONATE-->1-(2-Cyanostyryl)-4-(4-cyanostyryl)benzene-->Disodium 4,4'-bis(2-sulfostyryl)biphenyl |
|



