|
| | N,N-Dibenzylhydroxylamine Basic information |
| | N,N-Dibenzylhydroxylamine Chemical Properties |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-37/39 | | WGK Germany | 3 | | TSCA | Yes | | HS Code | 2928.00.2500 |
| | N,N-Dibenzylhydroxylamine Usage And Synthesis |
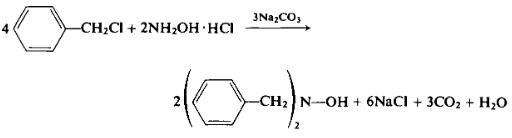
| Chemical Properties | white to slightly yellow adhering crystalline | | Uses | N,N-Dibenzylhydroxylamine, upon oxidation, yields N-benzyl-α-phenylnitrone, which can undergo cycloaddition reaction with suitable dipolarophiles. It can be used to synthesize N,N,O-trisubstituted hydroxylamines and arylamines. | | Preparation | A mixture of 14 gm (0.202 mole) of hydroxylamine hydrochloride and 50 gm (0.395 mole) of benzyl chloride in 200 ml of 70% ethanol is treated with 60 gm of crystalline sodium carbonate. The mixture is heated under a reflux condenser for 2 hr, cooled to room temperature, filtered, and the solids discarded. The filtrate is treated with sufficient ice water to cause precipitation of A^N-dibenzylhydroxylamine. The reaction mixture is then thoroughly cooled in a freezing mixture to permit complete precipitation of product to take place. The yield, upon filtration, is 26 gm (61.5%), m.p. 123°C. A similar preparation has recently been reported [13a].
 |
| | N,N-Dibenzylhydroxylamine Preparation Products And Raw materials |
|



