|
| | TOFOGLIFLOZIN Basic information |
| Product Name: | TOFOGLIFLOZIN | | Synonyms: | Tofogliflozin (hydrate);(1S,3'R,4'S,5'S,6'R)-6-[(4-Ethylphenyl)methyl]-3',4',5',6'-tetrahydro-6'-(hydroxymethyl)spiro[isobenzofuran-1(3H),2'-[2H]pyran]-3',4',5'-triol hydrate (1:1);Tofogliflozin hydrate (1:1);Tofogliflozin Monohydrate;Tofogliflozin hydrate (1:1)-API;CSG452; CSG-452; TOFOGLIFLOZIN; CSG 452; UNII-554245W62TTOFOGLIFLOZIN [INN]; TOFOGLIFLOZIN ANYHYDROUS;Tofogliflozin anyhydrous;UNII-554245W62TTofogliflozin [INN] | | CAS: | 1201913-82-7 | | MF: | C22H26O6.H2O | | MW: | 404.46 | | EINECS: | | | Product Categories: | API | | Mol File: | 1201913-82-7.mol |  |
| | TOFOGLIFLOZIN Chemical Properties |
| storage temp. | Store at -20°C | | solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; Ethanol: 30 mg/ml; Ethanol:PBS (pH7.2)(1:20): 0.05 mg/ml | | form | A crystalline solid |
| | TOFOGLIFLOZIN Usage And Synthesis |
| Description | Tofogliflozin hydrate, which is a sodium-glucose co-transporter
2 inhibitor, was approved in Japan for the treatment of type 2 diabetes
at the same time as luseogliflozin hydrate (XIX). The drug
was discovered by Chugai Pharmaceutical and jointly developed
with Sanofi-Aventis and Kowa. Tofogliflozin hydrate reduces
glucose levels by inhibiting the reuptake of glucose by selectively
inhibiting SGLT2, and plays a key role in the reuptake of glucose
in the proximal tubule of the kidneys. | | Synthesis | Reduction of commercially available 2-bromoterephtalic acid
(268) through the use of trimethoxyborane and borane-
THF proceeded in 89% yield to afford diol 269. Subjection of
this compound to 2-methoxypropene (270) under acidic
conditions generated bis-acetonide 271. This bromide then
underwent lithium¨Chalogen exchange followed by exposure to
magnesium bromide and treatment with lactone 272 (which
was prepared by persilylation of commercially available
(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2Hpyran-
2-one (277). This mixture was worked up with
aqueous ammonium chloride and upon treatment with p-TsOH
in methanol resulted in spiroacetal 273. Next, global protection
of all alcohol functionalities within 273 was affected by reaction
with methylchloroformate and DMAP in acetonitrile. The benzyl
carbonate within 274 was selectively exchanged via Suzuki coupling
with 4-ethylphenylboronic acid (275) to afford methylene
dibenzyl system 276. Subsequent treatment with aqueous sodium
hydroxide in methanol followed by crystallization from 1:6 acetone
and water furnished the desired product tofogliflozin hydrate
(XXXIV) in 75% yield. 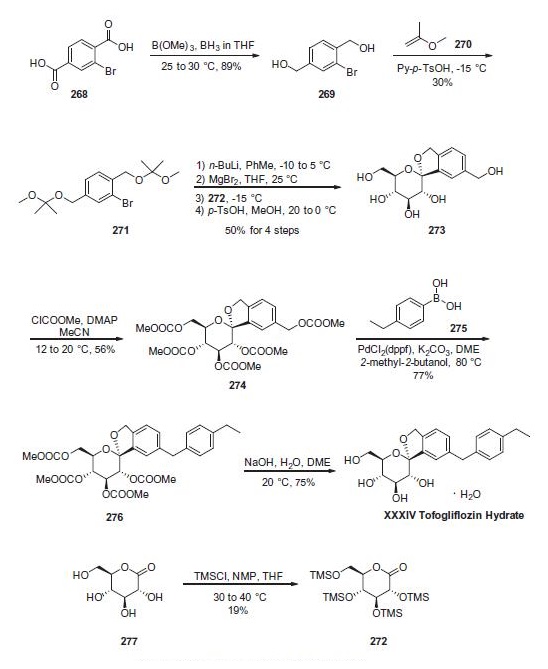
|
| | TOFOGLIFLOZIN Preparation Products And Raw materials |
|