|
| | Memantine HCl Chemical Properties |
| Melting point | 292 °C | | storage temp. | Inert atmosphere,2-8°C | | solubility | Soluble in Water (up to 20 mg/ml) | | form | Fine Powder | | color | White | | Water Solubility | Soluble in water (100mM) | | Merck | 14,5806 | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in distilled water may be stored at -20°C for up to 2 months. | | CAS DataBase Reference | 41100-52-1(CAS DataBase Reference) |
| Safety Statements | 24/25 | | WGK Germany | 3 | | HazardClass | IRRITANT | | HS Code | 29213000 |
| | Memantine HCl Usage And Synthesis |
| Description | Memantine HCl is the HCl form of Memantine. Memantine is a well- known neuroprotective drug used for the treatment of Alzheimer’s disease. It is believed to be the first neuroprotective drug that has achieve clinical approval by US FDA as well as European. The development of Alzheimer’s disease involves the misfolded mutant proteins such as β-amyloid peptide (Aβ, particularly Aβ1−42). These misfolded mutant protein can increase the NMDA response and further cause severe excitotoxicity. Memantine suppresses this process through acting as the antagonist of the N-methyl-d- aspartate-sensitive glutamate receptor (NMDARs). Memantine is also under investigation as a potential treatment for other kind of neurodegenerative disorders such as HIV-associated dementia, neuropathic pain, glaucoma, depression, Huntington’s disease, ALS and movement disorders, among other.
| | References | Reisberg, Barry, et al. New England Journal of Medicine 348.14 (2003): 1333-1341.
Lipton, Stuart A. Nature reviews Drug discovery 5.2 (2006): 160-170.
| | Description | Memantine, a NMDA receptor antagonist, was
co-developed by Forest Laboratories with Merz
Pharmaceuticals and marketed under the trade name
Namenda for the treatment of Alzheimer’s disease in the US
after its approval in October, 2003. This drug has been
available in many European and Asian markets before
getting approval in the US. | | Description | Memantine is an NMDA receptor antagonist that blocks NMDA-induced currents in rat retinal ganglion cells by 90% when used at a concentration of 12 μM. It reverses inhibition of dephosphorylation of the synthetic tau phosphopeptide p17 (tau194-207) induced by the endogenous inhibitor of protein phosphatase 2A (PP2A) I1PP2A in vitro. In vivo, memantine (2 mg/kg) restores PP2A activity, decreases GSK-3β and amyloid-β (Aβ) levels in the hippocampus, cerebral cortex, and ventricular areas, and attenuates spatial learning and memory in the AAV1-I1PP2A rat model of Alzheimer''s disease. Memantine (20 mg/kg) reduces responding on the ethanol-associated lever in a cue-induced ethanol-seeking test in rats. It also decreases secretion of matrix metalloproteinase-9 (MMP-9), degradation of collagen IV, the size of cerebral ischemia-induced brain infarcts, and neuronal cell death in a mouse model of focal cerebral ischemia. | | Chemical Properties | Crystalline Solid | | Uses | Used as an antiparkinsonian and antispasmodic | | Uses | Antiparkinsonian;Dopaminergic agonist | | Uses | Anti Alzheimer's | | Application | Memantine HCl has been used:- as a media
supplement to serve as a control to study the neuroprotective effects of
erythropoietin in N-methyl-D-aspartate receptor-induced toxicity.
- to intraperitoneally inject experimental animal to study its effect on traumatic injury.
- as a test compound to determine the dynamic range and selectivity of retinal pigment epithelium tissue penetration.
| | Definition | ChEBI: A hydrochloride obtained by reaction of memantine with one equivalent of hydrochloric acid. A low to moderate affinity uncompetitive (open-channel); NMDA receptor antagonist which binds preferentially to the NMDA receptor-operated cation channels. | | Brand name | Namenda(Forest). | | General Description | Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Memantine Hydrochloride is a drug used for treating the Alzheimer′s disease by means of reducing abnormal activity in the brain. It can help patients suffering from dementia as it assists them to think more clearly and perform their chores with ease. | | Biochem/physiol Actions | Memantine is effective against Alzheimer′s disease and Parkinson′s disease. It is also useful in treating epilepsy, motor neurone disease and trauma. | | Synthesis | Memantine (XV) or 1-amino-
3,5-dimethyladamantane hydrochloride was first synthesized
by Lilly as an anti-diabetic agent but was ineffective in
lowering blood sugar. Several syntheses have been
detailed in the literature. However the simplest
synthesis of the drug was done in one step from the
commercially available 3,5-dimethyl adamantine (122).
Treatment of 122 with nitrogen trichloride (CAUTION: very
explosive gas!) in the presence of aluminum trichloride (ratio
of 1.5:1.2) gave the desired amino adamantine in 86% yield.
However, a much safer alternative has been reported by Lilly
scientists. Heating the commercially available 3,5-dimethyladamantane 122 in bromine gave the bromo
derivative 123 (86%) which was then reacted with sulfuric
acid in acetonitrile to provide quantitatively acetyl amino
derivative 124 after aqueous workup. Hydrolysis of the
acetyl group was done by heating 124 with sodium
hydroxide in diethylene glycol to give 1-amino -3,5-
adamantane (96%), which was then made into the
hydrochloride salt in ether and recrystallized from ether and
alcohol mixture to provide the final product memantine
hydrochoride XV. 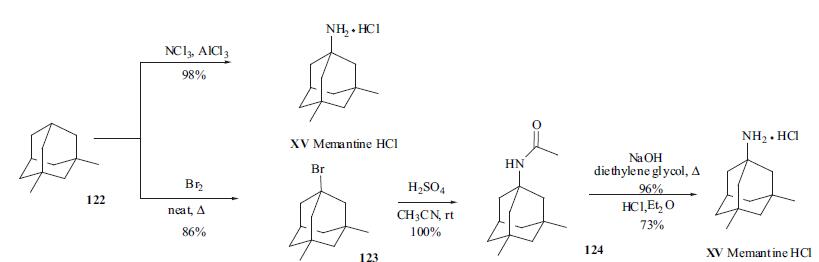
| | storage | Room temperature | | References | 1) Chen and Lipton (1997), Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism; J. Physiol. 499 27
2) Chen et al. (1998), Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation; Neuroscience 86 1121
3) Lipton et al. (2005), The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: low-affinity, uncompetitive antagonism; Curr. Alzheimer. Res. 2 155
4) Parsons et al. (1999), Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist—a review of preclinical data; Neuropharmacology 38 735
5) Witt et al. (2004), Memantine hydrochloride; Nat. Rev. Drug Discov. 3 109 |
| | Memantine HCl Preparation Products And Raw materials |
|



