| description | Methimazole is an antithyroid medication used in the therapy of hyperthyroidism and Graves disease. It works by making it harder for the body to make thyroid hormone.
It is also known as thiamazole, is a thioamide and a thyroid hormone antagonist which acts by inhibiting the incorporation of iodine into tyrosyl residues of thyroglobulin and, thus, lowering thyroid hormone levels.
It resembles propylthiouracil both in chemical structure and activity.
Methimazole is primarily used for the treatment of hyperthyroidism, by inhibiting the peroxidase system, hindering thyroxine (T4) and tri-triiodothyronine (T3) synthesis, animal experiments show that it can inhibit B lymphocytes synthesizing antibodies, and reduce levels of thyroid stimulating antibodies in blood circulating it can make suppressing T cell function return to normal, it is applicable to hyperthyroidism caused by a variety of factors . Like other various drugs (propylthiouracil), it also has some adverse reactions while it is taken, Including hematologic adverse reactions, primarily neutropenia, hematopoietic dysfunction or disorder, thrombocytopenia, reduction of prothrombin or factor VII ; long-term medication can also cause liver damage,such as cholestatic jaundice and toxic hepatitis ; other skin reactions such as hair loss, itching, rash, dermatitis, lupus erythematosus, as well as some other rare adverse reactions. Also methimazole allows prothrombin time to prolong , and increases serum alkaline phosphatase, aspartate aminotransferase (AST) and alanine aminotransferase (ALT)and causes blood bilirubin and blood lactate dehydrogenase increasing. Therefore, patients in the medication should regularly take blood tests, liver function tests and peripheral blood leukocytes.
The above information is edited by the Chemicalbook of Tian Ye. |
| Chemical properties | leaf-shaped crystalline (ethanol), melting point 146-148 ℃, boiling point 280 ℃ (decomposition), soluble in water, soluble in alcohol, chloroform, slightly soluble in ether, benzene.
|
| Uses | Carbimazole intermediates.
anti-thyroid drugs.
|
| Production methods | React amino acetal with methyl isothiocyanate to genarate this product. The product can also be produced from thiocyanate and N-substituted amino acetal.
|
| Chemical Properties | White Solid |
| Originator | Favistan ,Asta |
| Uses | Methimazole also directly disrupts thyroxine and triiodothyronin sysnthesis in the thyroid
gland, and it is used for the same indications as propylthiouracil and methylthiouracil to
treat hyperfunctioning thyroid glands in patients with Basedow’s disease. |
| Uses | 2-Mercapto-1-methylimidazole was employed as hydrophobic charge-induction chromatography ligand for antibody purification. It was also used in preparation of nitrile functionalized methimazole-based room temperature ionic liquids. |
| Uses | Methimazole is a thiourea antithyroid agent that prevents iodine organification, thus inhibiting the synthesis of thyroxine. Antihyperthyroid |
| Definition | ChEBI: Methimazole is a member of the class of imidazoles that it imidazole-2-thione in which a methyl group replaces the hydrogen which is attached to a nitrogen. It has a role as an antithyroid drug. |
| Manufacturing Process | 2 Methods of preparation of thiamazole:
1. To 2,2-diethoxyethylamine methylisothiocyanate was added and mixed after then 1-(2,2-diethoxy-ethyl)-3-methylthiourea was obtained.
The reaction of the 1-(2,2-diethoxyethyl)-3-methylthiourea with sulfuric acid yield thiamazole.
2. 1,1-Diethoxyethane was treated by bromine in the presence CaCO3 and 2- bromo-1,1-diethoxyethane was obtained.
Then to the 2-bromo-1,1-diethoxyethane methylamine was added, mixed and reaction mixture was heated to 120°-130°C in autoclave. As the result (2,2- diethoxyethyl)methylamine was obtained.
(2,2-Diethoxyethyl)methylamine reacted with potassium thiocyanate in the presence of hydrochloric acid and give the thiamazole, yellow crystallic precipitate, melting point 144°-147°C. |
| Brand name | Tapazole (Jones);
Tapazole (King)
. |
| Therapeutic Function | Thyroid inhibitor |
| General Description | Methimazole, 1-methylimidazole-2-thiol (Tapazole), occurs as a white to off-white, crystallinepowder with a characteristic odor and is freely soluble inwater. A 2% aqueous solution has a pH of 6.7 to 6.9. It shouldbe packaged in well-closed, light-resistant containers. |
| Biochem/physiol Actions | Methimazole is a thiourea antithyroid agent that prevents iodine organification, thus inhibiting the synthesis of thyroxine. |
| Clinical Use | Methimazole is indicated in the treatment of hyperthyroidism.It is more potent than propylthiouracil. The side effectsare similar to those of propylthiouracil. As with otherantithyroid drugs, patients using this drug should be undermedical supervision. Also, like the other antithyroid drugs,methimazole is most effective if the total daily dose is subdividedand given at 8-hour intervals. |
| Safety Profile | Poison by
subcutaneous route. Moderately toxic by
ingestion and intraperitoneal routes. Human
teratogenic effects. An experimental
teratogen. Experimental reproductive
effects. Questionable carcinogen with
experimental neoplastigenic data. Human
mutation data reported. An antithyroid drug.
When heated to decomposition it emits very
toxic fumes of NOx and SOx. See also
MERCAPTANS. |
| Synthesis | Methimazole, 1-methyl-2-imidazolthiol (25.2.5), is synthesized by reacting
aminoacetic aldehyde diethylacetal with methylisothiocyanate and subsequent hydrolysis
of the acetal group of the resulting disubstituted urea derivative 25.2.4 by a solution
of sulfuric acid, during which a simultaneous cyclization reaction takes place, forming the
imidazole ring of the desired methimazole . 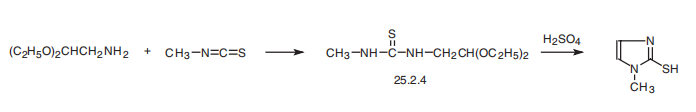
|
| Veterinary Drugs and Treatments | Methimazole is considered by most clinicians to be the agent
of choice when using drugs to treat feline hyperthyroidism.
Propylthiouracil has significantly higher incidences of adverse
reactions
when compared to methimazole and is rarely used today.
Transdermal methimazole (in PLO gel; 2.5 mg twice daily) has been
used with some therapeutic success in cats that do not tolerate oral
dosing. Efficacy may require four or more weeks to detect. Studies
are ongoing.
Methimazole appears to be useful for the prophylactic prevention
of cisplatin induced nephrotoxicity in dogs. |
| Purification Methods | Crystallise it from EtOH. UV: at 251nm (H2O), 260nm (EtOH) and 267nm (CHCl3). [Lawson max & Morley J Chem Soc 1103 1956, Beilstein 24 H 17, 24 III/IV 61.] |



