|
| | Isobornyl methacrylate Basic information |
| Product Name: | Isobornyl methacrylate | | Synonyms: | 2-methyl-,1,7,7-trimethylbicyclo(2.2.1)hept-2-ylester,exo-2-propenoicaci;IBOMA;Isobornyl methacrylate ,98%;Isobornyl Methacrylate ;Isobornyl Methacrylate, stabilized, 85-90% 50GR;(1R,2R,4R)-1,7,7-TriMethylbicyclo[2.2.1]heptan-2-yl Methacrylate;Isobornyl Methacrylat;Isobornyl Methacrylate technical grade | | CAS: | 7534-94-3 | | MF: | C14H22O2 | | MW: | 222.33 | | EINECS: | 231-403-1 | | Product Categories: | Bicyclic Monoterpenes;Biochemistry;Terpenes;7534-94-3 | | Mol File: | 7534-94-3.mol |  |
| | Isobornyl methacrylate Chemical Properties |
| Melting point | -60 °C | | Boiling point | 127-129 °C15 mm Hg(lit.) | | density | 0.983 g/mL at 25 °C(lit.) | | vapor pressure | 7.5Pa at 20℃ | | refractive index | n20/D 1.477(lit.) | | Fp | 225 °F | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | form | Liquid | | color | Clear colorless to yellow | | Specific Gravity | 0.985 | | Water Solubility | negligible | | InChIKey | HHHKSPVBHWRWNA-QOZQQMKHSA-N | | LogP | 5.09 | | CAS DataBase Reference | 7534-94-3(CAS DataBase Reference) | | EPA Substance Registry System | Isobornyl methacrylate (7534-94-3) |
| | Isobornyl methacrylate Usage And Synthesis |
| Chemical Properties | CLEAR COLORLESS TO YELLOW LIQUID | | Characteristics | Isobornyl methacrylate is a colorless transparent liquid. It is a monomer that unifies hardness and flexibility. Due to its molecular structure, its polymer has excellent high gloss, sharp image, scratch resistance, medium resistance and weather resistance, and Its hygroscopicity is significantly lower than that of MMA (methyl methacrylate). In addition, IBOMA-added acrylic resin has good compatibility with polyester, alkyd and many volatile paint film-forming substances. | | Uses | Isobornyl methacrylate is a versatile hydrophobic monomer with a good combination of hardness and flexibility that can improve the chemical and water resistance of polymer systems. | | Preparation | Isobornyl methacrylate was synthesized by direct esterification of camphene and methacrylic acid. | | General Description | Isobornyl methacrylate is a reactive solvent with low vapor pressure. It facilitates the free radical polymerization by forming a polymer with high glass transition temperature (Tg). | | Flammability and Explosibility | Notclassified | | Synthesis | Isobornyl methacrylate is obtained by reacting (1S,2S,4S)-(+)-isoborneol and 4-methoxyphenol.Dissolve5g (1S,2S,4S)-(+)-isoborneol in 15 mL toluene. Add 4 mg (0.032 mmol) 4-methoxyphenol to the solution. After the addition of 0.4 g (3.24 mmol)4-dimethylamino pyridine, add a solution of 10 g (64.8 mmol) methacrylic anhydride (MAA) in 10 mL toluene dropwise to the mixture. Heat the reaction mixture to 80 °C. Stir the reaction mixture for 16 hours. After cooling down to room temperature, wash the mixture with 50 mL 1M sodium bicarbonate solution four times. Wash the organic phase with 50 mL brine two times. Separate the organic phase. Remove the toluene by rotary evaporation followed by a fractional distillation under reduced pressure.
1H NMR (CDCl3, 300 MHz): δ(ppm) = 6.06 (dq, 1H, 1); 5.51 (dq, 1H, 2); 4.71 (m ,1H, 4); 1.93 (dd, 3H, 3); 1.87-1.5 (m, 5H, 6,7,8); 1.23-1.04 (m, 2H, 5); 1.012 (s, 3H, 9);0.86 (s, 3H, 10); 0.85 (s, 3H, 11). IR (film): ν(cm-1) = 2954 (CH2), 2879 (CH2), 1715 (C=O) 1454 (CH2), 1157 and 1053 (C=O).
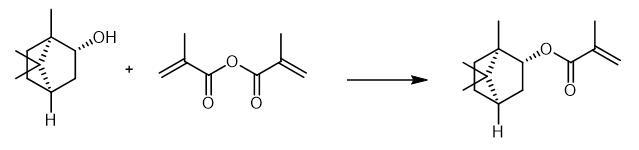
Fig The synthetic method of Isobornyl methacrylate |
| | Isobornyl methacrylate Preparation Products And Raw materials |
|