|
| | Succimer Basic information |
| Product Name: | Succimer | | Synonyms: | (2R,3S)-2,3-diMercaptosuccinic acid;(2R,3S)-rel-2,3-DiMercaptosuccinic acid;Butanedioic acid,2,3-diMercapto-, (2R,3S)-rel-;meso-2,3-Dimercaptosuccinic acid;API Succimer;-rel-2,3-Dimercaptosuccinic acid;(2S,3R)-2,3-dimercaptobutanedioate;(r*,s*)-2,3-dimercaptobutanedioicacid | | CAS: | 304-55-2 | | MF: | C4H6O4S2 | | MW: | 182.22 | | EINECS: | 206-155-2 | | Product Categories: | API;Inhibitors;Organic acids;Analytical Chemistry;Ligands for Pharmaceutical Research;Radiopharmaceutical Chemistry (Chelating Reagents);Building Blocks;Chemical Synthesis;Organic Building Blocks;Sulfur Compounds;Thiols/Mercaptans;BDO;304-55-2 | | Mol File: | 304-55-2.mol |  |
| | Succimer Chemical Properties |
| Melting point | 196-198 °C (dec.)(lit.) | | Boiling point | 285.62°C (rough estimate) | | density | 1.439 (estimate) | | refractive index | 1.5220 (estimate) | | storage temp. | -20°C | | solubility | DMSO (Sparingly), Methanol (Slightly, Heated) | | pka | pK1:2.71;pK2:3.48;pK3:8.89(SH);pK4:10.79(SH) (25°C) | | form | Powder | | color | White to off-white | | Water Solubility | Soluble in water, and ethanol (25 mg/ml, results in colorless to light yellow, clear). | | Merck | 14,8865 | | BRN | 1725150 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. | | InChIKey | ACTRVOBWPAIOHC-XIXRPRMCSA-N | | CAS DataBase Reference | 304-55-2(CAS DataBase Reference) | | EPA Substance Registry System | Butanedioic acid, 2,3-dimercapto-, (2R,3S)-rel- (304-55-2) |
| | Succimer Usage And Synthesis |
| Description | Succimer is a newly available oral chelator of lead and other heavy metals. The
compound is indicated for the treatment of lead poisoning in children. Succimer does
not bind iron, calcium or magnesium and preferentially binds lead, mercury and arsenic
over copper or zinc. The metal-bound succimer is excreted in the urine. The drug may
also be effective in adults. | | Chemical Properties | white or off-white powder | | Originator | Johnson and Johnson (J&J) (U.S.A.) | | Uses | chelating agent, antihypertensive | | Uses | meso-2,3-Dimercaptosuccinic Acid is used as a chelating agent and masking agent for cadmium in EDTA titration of zinc. | | Uses | chelating agent, treatment of lead poisoning | | Uses | A water-soluble chelating agent. | | Definition | ChEBI: A sulfur-containing carboxylic acid that is succinic acid bearing two mercapto substituents at positions 2 and 3. A lead chelator used as an antedote to lead poisoning. | | Preparation |
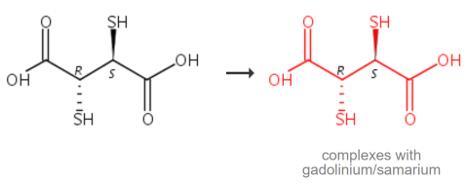
Isolate
the solid complexes of trivalent lanthanide complexes with
meso-2,3-Dimercaptosuccinic Acid, furan-2-carboxylic acid,
meso-2,3-dimercaptosuccinic acid and sarcosine from the mixture of
equimolar solutions of metal nitrates and ligands. Adjust the pH of the
mixture to 7 by adding dilute solution of KOH. Reflux the mixture in
ethanol (15-20 ml) for 3-4 hours on a steam bath. The clear solution
gives a solid mass on cooling, filter through G4 glass crucible and wash
several times with the mixture of doubly distilled water and alcohol.
Recrystallise it to obtain pure crystal and dry at 60 ?? -70 ??C [1].
| | Brand name | Chemet (Ovation). | | Pharmaceutical Applications | Dimercaptosuccinic acid (DMSA) is a modification of BAL containing two thiol groups, which are responsible for the unpleasant
smell, and two carboxylic acid groups. DMSA is also known under the name Succimer. It chemical name
is meso-2,3-dimercaptosuccinic acid and the chemical formula is HO2CCH(SH)CH(SH)CO2H. There are
two diastereomeric forms, meso and the chiral DL forms, with the meso isomer being used as chelating agent.
DMSA was developed in the 1960s and replaced BAL and edetate in some countries for the treatment
of lead, arsenic and mercury poisoning. Furthermore, the dimethylester modification of DMSA has been
successfully used for the treatment of heavy-metal poisoning. | | Pharmaceutical Applications | The unlicensed drug Succimer (DMSA, meso-2,3-dimercaptosuccinic acid) may be valuable in the treatment
of most forms of heavy-metal poisoning including lead, arsenic and mercury. These and other chelating
agents such as unithiol (DMPS, 2,3-dimercapto-1-propanesulfonic acid) and α-lipoic acid (ALA) are
also used in alternative medicine, which has led to much criticism and discussion. So far, no medical
study has proven the effectiveness of chelation therapy for any clinical application other than heavy-metal
poisoning. | | Veterinary Drugs and Treatments | In veterinary medicine, succimer may be useful for the oral
treatment of lead poisoning in small animals (including birds).
Potentially, it also may be of benefit for the treatment of other toxic
heavy metals such as arsenic or mercury, but more research must be
done before this can be recommended. | | Purification Methods | Purify the acid by dissolving it in NaOH and precipitating with dilute HCl, drying and recrystallising from MeOH. IR has at 2544 (SH) and max 1689 (CO2H) cm-1 . The bis-S-acetyl derivative has m 183-185o (from EtOAc or Me2CO), and its Me ester has m 119-120o (from pet ether) [Gerecke et al. Helv Chim Acta 44 957 1961, Owen & Sultanbawa J Chem Soc 3112 1949]. [Beilstein 3 III 1033.] |
| | Succimer Preparation Products And Raw materials |
|



