|
| | Iguratimod Basic information |
| Product Name: | Iguratimod | | Synonyms: | IGURATIMOD;iguratimod( R&D);T 614;N-[7-methanesulfonamido-4-oxo-6-(phenoxy)chromen-3-yl]formamide;3-(Formylamino)-7-(methylsulfonylamino)-6-phenoxy-4H-1-benzopyran-4-one;N-[3-(Formylamino)-4-oxo-6-phenoxy-4H-1-benzopyran-7-yl]methanesulfonamide;IguratiMod (T 614);N-(7-(MethylsulfonaMido)-4-oxo-6-phenoxy-4H-chroMen-3-yl)forMaMide | | CAS: | 123663-49-0 | | MF: | C17H14N2O6S | | MW: | 374.37 | | EINECS: | 808-127-0 | | Product Categories: | Pharmaceutical intermediate;Pharmaceuticals;API;123663-49-0 | | Mol File: | 123663-49-0.mol |  |
| | Iguratimod Chemical Properties |
| Melting point | 238.0 to 242.0 °C | | Boiling point | 580.6±60.0 °C(Predicted) | | density | 1.52±0.1 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | solubility | DMSO (Slightly) | | pka | 5.58±0.20(Predicted) | | form | Solid | | color | White to Off-White |
| | Iguratimod Usage And Synthesis |
| Description | In August 2011, China’s State FDA approved Simcere Pharmaceutical
Group’s new drug application for iguratimod (T-614), a disease modifying
anti-rheumatic drug (DMARD) for the treatment of rheumatoid arthritis
(RA). Preclinical in vivo studies indicated that iguratimod was effective
in an established adjuvant-induced arthritis model (ED40=3.6 mg/kg)
in rats and also efficacious in a type II collagen-induced arthritis model in DBA/1J mice at 30 mg and 100 mg/kg. | | Originator | Toyama (Japan) | | Uses | Iguratimod acts as an anti-inflammatory agent, used primarily in the treatment of rheumatoid arthritis. | | Definition | ChEBI: Iguratimod is an organic molecular entity. | | Brand name | Iremod | | Clinical Use | Iguratimod, which was discovered by Toyama Pharmaceuticals and jointly co-developed with Eisai in
Japan, was approved by the PMDA (Pharmaceuticals and Medical Devices Agency) of Japan on June 29,
2012 for the treatment of rheumatoid arthritis. This drug was also independently developed by
Simcere Pharmaceutical Group and is marked as Iremod® in China. The drug exhibited inhibitory
effects on granuloma inflammation, and was shown to be efficacious for the prevention of joint
destruction in adjuvant arthritis. | | Synthesis | Several synthesis of iguratimod have been published, the
most likely scale synthesis, which does not require chromatographic purification, is described in the scheme.The synthesis began with commercially available 3-nitro-4-chloro anisole (78) which was reacted
with potassium phenoxide (generated from phenol and potassium t-butoxide at 110 oC) to provide the
corresponding nitrophenyl ether which was subsequently reduced and sulfonylated to furnish
sulfonamide 79. Next, this diphenyl ether was subjected to a Friedel-Crafts reaction with
aminoacetonitrile hydrochloride which gave rise to aminomethylacetophenone 80 in 90% yield. This
aminoketone was then formylated with formic trimethylacetic anhydride 81 at room temperature to
afford formamide 82 in 91% yield, and this material was immediately subjected to O-demethylation
conditions with aluminum trichloride and sodium iodide in acetonitrile to give the phenol 83 in 95%
yield. Finally, treatment of the aminomethyl acetophenone phenol 83 with N,N-dimethylformamide
dimethylacetal in DMF at low temperatures furnished iguratimod (XII) in 87% yield. 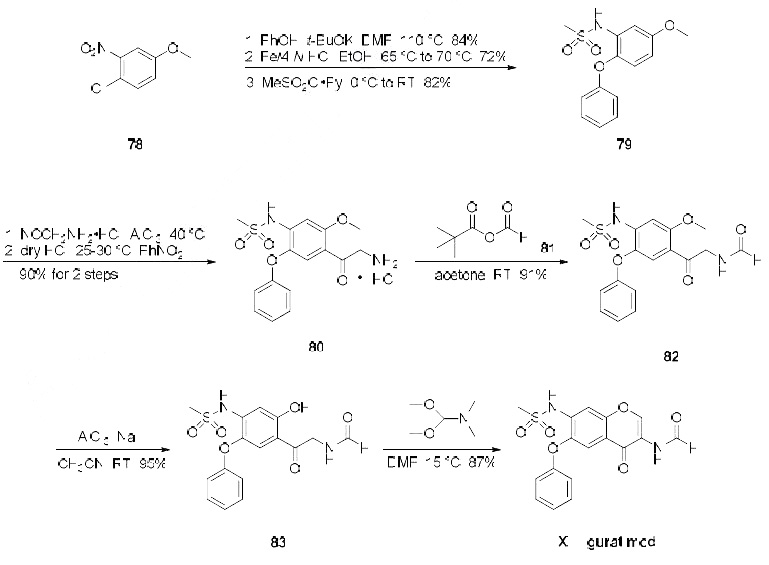
| | in vitro | iguratimod inhibited the release of immunoreactive il-1 beta from human monocytic cell line stimulated with lipopolysaccharides (lps) in a dose-dependent manner (0.3-30 μg/ml). northern blotting analysis using lps-stimulated thp-1 cells indicated that the inhibitory effect of iguratimod on il-1 beta production is caused by the suppression of il-1 beta mrna expression [1]. | | in vivo | administration of iguratimod did not inhibit the tumor growth, but resulted in attenuation of cachexia symptoms. furthermore, iguratimod decreased the serum levels of il-6, and also reduced its gene expression in the tumor tissues. in addition, exogenously administered il-6 nullified the suppressive effect of iguratimod [2]. | | target | COX-2 | | IC 50 | 2.0 (hepatocyte-stimulating activities) and 6.6 μg/ml (immunoreactivities) for il-6 release. | | references | [1] tanaka k, aikawa y, kawasaki h, asaoka k, inaba t, yoshida c. pharmacological studies on 3-formylamino-7-methylsulfonylamino-6-phenoxy-4h-1-benzopyran-4-one (t-614), a novel antiinflammatory agent. 4th communication: inhibitory effect on the production of interleukin-1 and interleukin-6. j pharmacobiodyn. 1992;15(11):649-55.
[2] tanaka k, urata n, mikami m, ogasawara m, matsunaga t, terashima n, suzuki h. effect of iguratimod and other anti-rheumatic drugs on adenocarcinoma colon 26-induced cachexia in mice. inflamm res. 2007;56(1):17-23.
[3] hara m, abe t, sugawara s, mizushima y, hoshi k, irimajiri s, hashimoto h, yoshino s, matsui n, nobunaga m. long-term safety study of iguratimod in patients with rheumatoid arthritis. mod rheumatol. 2007;17(1):10-6. |
| | Iguratimod Preparation Products And Raw materials |
|