|
| | Blonanserin Chemical Properties |
| Melting point | 117-119°C | | Boiling point | 540.8±50.0 °C(Predicted) | | density | 1.095±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO: ≥10mg/mL | | pka | 7.66±0.10(Predicted) | | form | powder | | color | white to off-white | | Merck | 14,1319 |
| Hazard Codes | Xn | | Risk Statements | 22 | | WGK Germany | 1 | | RTECS | GY0175140 | | HS Code | 29339900 | | Toxicity | mouse,LD,oral,> 500mg/kg (500mg/kg),United States Patent Document. Vol. #5021421, |
| | Blonanserin Usage And Synthesis |
| Outline | This product is a serotonin and dopamine antagonist (SDA), it is a second-generation atypical antipsychotic drugs, and has a considerable dopamine D2 receptor blocking action as haloperidol , it also has a strong 5-HT2A blocking effect, the selectivity on both receptors is stronger than other antipsychotics. It displays fewer extrapyramidal response side effects than conventional antipsychotics on the market . It is developed and sold by Sumitomo Pharmaceutical Co., Ltd. of Japan. Listing: April 2008 in Japan.
| | Synthesis method | Fluorobenzoate condensates with acetonitrile to get fluorobenzoyl acetonitrile, prepared in polyphosphoric acid by the reaction of 3-(4-fluorophenyl)-3-oxo-propionamide, and cyclooctanone cyclization of 4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-cyclooctane and [b] pyridin-2 (1H)-one, and recrystallized from benzene dichloride Lin acid dichloride with N-ethylpiperazine nucleophilic substitution to give antipsychotic blonanserin, the overall yield is about 42%.
Author: Junfang Wang, Xiaomei Wang , Zhefeng Wang , Huilin Shi
Author unit: Shanghai Institute of Pharmaceutical Industry
Journal Name: Chinese Journal of Pharmaceuticals
2009 40 (4)
| | Atypical antipsychotic drugs | Schizophrenia is a common psychiatric disease, since the early 1950s, for the discovery of antipsychotic effects of chiorpromazinum. Schizophrenia has been treated with drugs.
According to receptor blocking actions, the commonly used antipsychotics are divided into two categories, naming the typical and atypical: typical antipsychotics are represented by chlorpromazine and haloperidol . The main mechanism is blocking dopamine receptors, it has a good effect on positive symptoms of schizophrenia (hallucinations, delusions, agitation, impulsive behavior, etc.) . Meanwhile extrapyramidal effects (EPS) are common, while the effects for negative symptoms (apathy, poverty of thought, will decline, etc.) are poor, while atypical antipsychotics show a wider spectrum for the treatment of negative symptoms better than traditional medicine, and it is safe, with more mild side effects, the dosage is smaller, there have been many more advanced forms, which greatly improve patient compliance, representative drugs include clozapine, risperidone, olanzapine .
Double-blind clinical trials for 8 weeks for blonanserin comparison with haloperidol shown, 263 patients with schizophrenia were involved. 8-24mg/day blonanserin is at least effective as 4-12mg/day of haloperidol, it can improve the final grade improvement rate(FGIR) score (at least two drugs in patients with moderate improvement rate of 61.2% and 51.3%, respectively), less incidence of extrapyramidal side effects (52.7% and 75.4%).
The above information is edited by the chemicalbook of Tian Ye. | | China Patent Information | January 2008 blonanserin was developed and sold by Sumitomo Pharmaceutical Co., Ltd. of Japan. This product was compound patent in 1989 . The patent has expired.
At present, the domestic product is not imported, according to the "Patent Law" and "Regulations on Administrative Protection Drugs" , the drug patent risk does not exist in China, it does not have the conditions of application for administrative protection.
| | Uses | Mental
| | Description | Blonanserin, a dual antagonist of dopamine D2 and serotonin 5-HT2 receptors, was launched past year in Japan for the oral treatment of psychosis and schizophrenia. It is the latest entry in the class of atypical antipsychotic agents to reach the market. Blonanserin exhibits high affinity for D2 and 5-HT2 receptors (IC50 23.6 and 9.85 nM, respectively). Its affinity for D2 receptors is very close to that of haloperidol and risperidone, whereas the affinity for 5-HT2 receptors is about 7 times higher than that of haloperidol and 10 times lower than that of risperidone.Blonanserin is chemically derived in three steps starting with a polyphosphoric acid mediated condensation reaction of cyclooctanone with 4-fluorobenzoylacetonitrile. The resultant 4-fluorophenylcycloocta[b]pyridin-2-one intermediate is converted to the corresponding 2-chloro derivative by treatment with phosphoryl chloride and subsequently condensed with 1-ethylpiperazine to afford blonanserin. | | Chemical Properties | Pale Yellow Solid | | Originator | Sumitomo (Japan) | | Uses | Blonanserin is a novel atypical antipsychotic agent with potent dopamine D2 (Ki, 14.8 nM) and serotonin 5-HT2(Ki, 3.98 nM) receptors antagonist properties.
| | Uses | A 5-HT2 Serotonin receptor and D2 Dopamine receptor antagonist, used as an antipsychotic. | | Definition | ChEBI: Blonanserin is an organic molecular entity. | | Manufacturing Process | Preparation of 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-
hexahydrocyclooc ta[b]pyridine:
A mixture of 2-chloro-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta
[b]pyridine (2.0 g), N-ethylpiperazine (2.4 g), and potassium iodide (1.1 g) is
stirred at 170°C for 5 hours. After cooling, the reaction mixture is dissolved in
ethyl acetate and water. The organic layer is washed with water and extracted
with 5% hydrochloric acid. The extract is made alkaline with potassium
carbonate, and extracted with ethyl acetate. The extract is washed with water,
dried over anhydrous sodium sulfate, and concentrated under reduced
pressure.
(a) The residue is recrystallized from acetonitrile to give the desired product
(1.2 g), MP: 123°-124°C.
This product obtained in the above (a) is converted to the following salt
thereof by treating the product with various acids. | | Brand name | Lonasen | | Therapeutic Function | Antipsychotic | | Synthesis | The synthesis of blonanserin III has been described
in both the primary and patent literature in the following scheme.
Condensation of cyclooctanone (21) with 4-fluorobenzoyl
acetonitrile (20) in 75% polyphosphoric acid at 110 ??C provided
the fused cyclooctapyridone 22 in 64% yield. Reaction
of 22 with phenyldichlorophosphinic acid (23) at 170 ??C
gave chloride 24 in 76% yield, which was then reacted with
potassium idodide and 4-ethyl piperidine (25) at 170 ??C to
give blonanserin (III) in 47% yield after crystallization from
acetonitrile. 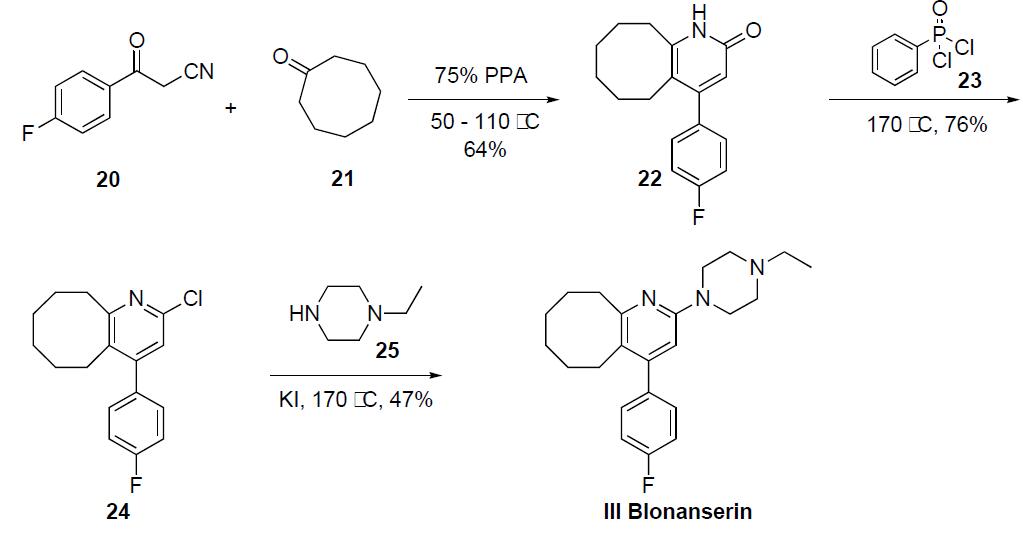
|
| | Blonanserin Preparation Products And Raw materials |
|



