|
| | 2,3-DIMETHOXYBENZONITRILE Basic information |
| | 2,3-DIMETHOXYBENZONITRILE Chemical Properties |
| Melting point | 68-70 °C(lit.) | | Boiling point | 290.25°C (rough estimate) | | density | 1.2021 (rough estimate) | | refractive index | 1.5300 (estimate) | | Fp | >230 °F | | storage temp. | Sealed in dry,Room Temperature | | solubility | Soluble in methanol. | | form | powder to crystal | | color | White to Light yellow | | BRN | 1451651 | | CAS DataBase Reference | 2024-83-1(CAS DataBase Reference) |
| Hazard Codes | Xn,T,Xi | | Risk Statements | 20/21/22-36/37/38 | | Safety Statements | 26-36 | | RIDADR | 3276 | | WGK Germany | 3 | | RTECS | DI4355000 | | HazardClass | 6.1 | | PackingGroup | III | | HS Code | 2926907090 |
| | 2,3-DIMETHOXYBENZONITRILE Usage And Synthesis |
| Chemical Properties | white to light yellow crystal powder | | Uses | 3,4-Dimethoxybenzonitrile is used as an organic chemical synthesis intermediate. | | Preparation | Veratraldoxime(88–89 g, 0.45 mol) was combined with acetic anhydride (100 g) in a 300-mL round-bottomed flask equipped with a ground-glass air condenser, and the mixture was cautiously heated. A vigorous reaction took place, at which point the flame (heating by oil bath; the author) was removed. After the reaction had subsided, the solution was gently refluxed for 2 h and then carefully poured, with stirring, into cold water (300 mL). Stirring was continued, and on cooling the nitrile separated as small, almost colorless crystals; these were collected by filtration and dried in air. The veratronitrile thus obtained was quite pure; yield 57–62 g (72–76%); mp 66–67 C°.
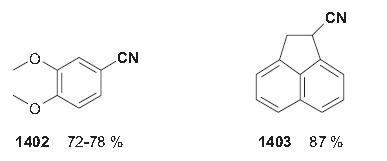
1-Cyanoacenaphthene 1403 can be synthesized from its carbaldoxime by dehydration with acetic anhydride in 87% yield. Whereas acetic anhydride usually serves as the solvent as well as the reactant, this procedure is performed in n-octane.
| | Synthesis Reference(s) | Synthesis, p. 510, 1985 DOI: 10.1055/s-1985-31253 |
| | 2,3-DIMETHOXYBENZONITRILE Preparation Products And Raw materials |
|



