|
| | (S)-Flurbiprofen Basic information |
| Product Name: | (S)-Flurbiprofen | | Synonyms: | (S)-(+)-FLURBIPROFEN;(S)-FLURBIPROFEN;(S)-(+)-2-FLUORO-ALPHA-METHYL-4-BIPHENYLACETIC ACID;Esflurbiprofen;(S)-2-fluoro-alpha-methyl[1,1'-biphenyl]-4-acetic acid;(S)-(+)-2-Fluoro-α-methyl-4-biphenylacetic acid;[1,1'-Biphenyl]-4-acetic acid, 2-fluoro-α-methyl-, (S)-;[1,1'-Biphenyl]-4-acetic acid, 2-fluoro-α-methyl-, (αS)- | | CAS: | 51543-39-6 | | MF: | C15H13FO2 | | MW: | 244.26 | | EINECS: | 257-263-1 | | Product Categories: | | | Mol File: | 51543-39-6.mol |  |
| | (S)-Flurbiprofen Chemical Properties |
| Melting point | 109-110 °C(lit.) | | Boiling point | 376.2±30.0 °C(Predicted) | | density | 1.199±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | insoluble in H2O; ≥10.9 mg/mL in DMSO; ≥69.1 mg/mL in EtOH | | form | A crystalline solid | | pka | 4.14±0.10(Predicted) |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 36/37/39-45 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 |
| | (S)-Flurbiprofen Usage And Synthesis |
| Description | Marketed by Taisho
Pharmaceutical Holdings Co., Ltd., and Teijin Pharma Ltd.,
the (S)-enantiomer of flurbiprofen (named Esflurbiprofen) was
approved as a new drug by the PMDA of Japan in 2015. (S)-
Flurbiprofen, a cyclooxygenase (COX)-inhibiting nonsteroidal
anti-inflammatory (NSAID) drug, is formulated with mentha
oil and administered by way of a patch as a treatment for
osteoarthritis in the current invention. Interestingly, the (R)-
enantiomer is responsible for minimal COX inhibition but has
been implicated as a possible treatment for a variety of other
diseases. Synthetic approaches to racemic flurbiprofen have been reported in the chemical literature as early as the 1960s,
and the mixture was approved as a drug in the United States by
the FDA in 1988, marketed by Pharmacia and Upjohn.
Toward this end, the current patent estate defines the utility of
the active S-enantiomer, and synthetic approaches therein apply
to this enantiomer only. | | Description | (S)-Flurbiprofen is the COX-active enantiomer of the non-selective COX inhibitor flurbiprofen with IC50 values of 0.48 and 0.47 μM for COX-1 and COX-2, respectively, in guinea pig whole blood. It inhibits the release of 6-keto prostaglandin F1α (6-keto-PGF1α; ) and thromboxane B2 (TXB2; ) from rat whole blood, gastric mucosa, lung, and jejunal tissue ex vivo in a dose-dependent manner. (S)-Flurbiprofen (1 nM) inhibits basal and bradykinin-, serotonin-, and histamine-stimulated prostaglandin E2 (PGE2) release from isolated skin flaps of rat lower hind paws. It also inhibits release of the neuroinflammatory marker calcitonin gene-related peptide (CGRP; Item Nos. 24405 | 24725 | 24728) when used at a concentration of 1 μM. In vivo, (S)-flurbiprofen reduces the number of flinches per minute in the formalin test in rats, indicating antinociceptive activity. | | Uses | (S)-Flurbiprofen is the S-isomer of Flurbiprofen (F598700), an anti-inflammatory used as an analgesic.
| | Definition | ChEBI: (S)-flurbiprofen is a flurbiprofen. It is an enantiomer of a (R)-flurbiprofen. | | Synthesis | The synthesis began with conversion of commercially
available aniline 168 to racemic flurbiprofen (169) through a Sandmeyer reaction and subsequent phenyl
group introduction through the use of sodium tetraphenylborate.
A chiral resolution was then performed on the resulting
stereogenic acid on multikilogram scale by treatment with (S)-
1-phenylethylamine in MeOH/toluene, which gave various
yields of salt 170 as reported by the authors. Acidification
with aqueous HCl delivered (S)-flurbiprofen (XXI). Importantly,
with respect to green chemistry considerations, the (R)-
enantiomer could be recycled by racemizing the undesired (R)-
enantiomer 171 in refluxing methanolic sulfuric acid, improving
the overall atom economy of the process and significantly
reducing waste. 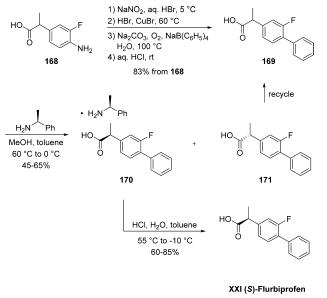
| | in vitro | it was found that the ic50 value of (s)-flurbiprofen was about 0.5 μm for both cox-1 and cox-2 in guinea pig whole blood [1]. | | in vivo | the efficacy of multiple applications of s(+)-flurbiprofen plaster (sfpp) was evaluated for the alleviation of inflammatory pain and edema in rat adjuvant-induced arthritis model. multiple applications of sfpp could exert a significant analgesic effect from the first day of application as compared to the other nsaid drugs. in terms of paw edema, sfpp could decrease edema from the second day after application. moreover, multiple applications of sfpp were found to be superior to those of other nsaid drugs in terms of the analgesic effect [2]. | | IC 50 | 0.5 μm for both cox-1 and cox-2 in guinea pig whole blood | | references | [1] barnett, j. ,chow, j.,ives, d., et al. purification, characterization and selective inhibition of human prostaglandin g/h synthase 1 and 2 expressed in the baculovirus system. biochimica et biophysica acta 1209, 130-139 (1994).
[2] sugimoto m et al. topical anti-inflammatory and analgesic effects of multiple applications of s(+)-flurbiprofen plaster (sfpp) in a rat adjuvant-induced arthritis model. drug dev res. 2016 jun;77(4):206-11.
[3] yataba i, otsuka n, matsushita i, matsumoto h, hoshino y. the efficacy and safety of s-flurbiprofen plaster in the treatment of knee osteoarthritis: a phase ii, randomized, double-blind, placebo-controlled, dose-finding study. j pain res. 2017 apr 11;10:867-880. |
| | (S)-Flurbiprofen Preparation Products And Raw materials |
|