|
| | Unii-4U07F515lg Basic information |
| Product Name: | Unii-4U07F515lg | | Synonyms: | Eltrombopag olamine;Sb 497115gr;Unii-4U07F515lg;3'-[(2Z)-2-[1-(3,4-DiMethylphenyl)-1,5-dihydro-3-Methyl-5-oxo-4H-pyrazol-4-ylidene]hydrazinyl]-2'-hydroxy-[1,1'-biphenyl]-3-carboxylic acid coMpd. with 2-aMinoethanol(1:2);EltroMbopag diethanolaMine salt;EltroMbopag OlaMine API;EltroMbopag IMpurity;Eltrombopag Diolamine | | CAS: | 496775-62-3 | | MF: | C27H29N5O5 | | MW: | 503.56 | | EINECS: | 629-876-8 | | Product Categories: | API;496775-62-3 | | Mol File: | 496775-62-3.mol |  |
| | Unii-4U07F515lg Chemical Properties |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | solubility | DMSO:56.33(Max Conc. mg/mL);99.76(Max Conc. mM)
DMF:1.0(Max Conc. mg/mL);1.77(Max Conc. mM)
DMF:PBS (pH 7.2) (1:3):0.25(Max Conc. mg/mL);0.44(Max Conc. mM)
Ethanol:0.1(Max Conc. mg/mL);0.18(Max Conc. mM) | | form | Powder | | Stability: | Hygroscopic |
| | Unii-4U07F515lg Usage And Synthesis |
| Description |
Eltrombopag is used to treat low blood platelet counts in adults with chronic immune (idiopathic) thrombocytopenia (ITP), when certain other medicines, or surgery to remove the spleen, have not worked well enough.Eltrombopag has also been recently approved (late 2012) for the treatment of thrombocytopenia (low blood platelet counts) in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
| | Uses | Treatment of chemotherapy-induced thrombocytopenia and treatment of immune thrombocytopenic purpura. | | Clinical Use | Eltrombopag olamine, a thrombopoietin receptor (TpoR) agonist,
was approved in late 2008 for the once-daily, oral short-term and long-term treatment of adult patients with previously treated
chronic idiopathic thrombocytopenic purpura (ITP). It is the first
small-molecule TpoR agonist and was launched in the U.S. for this
indication in 2009 by GlaxoSmithKline (GSK). Because eltrombopag
is a small molecule, the drug is administered orally and has
a reduced potential for causing an immune system reaction
versus alternative protein-based therapies. In 2010, eltrombopag
was approved in Europe for the long-term treatment of adult
patients with previously treated chronic ITP. | | Synthesis | The synthesis began
with the nitration of 2-bromophenol (39) with sodium nitrate
and sulfuric acid in water at 10??C to give 2-bromo-6-nitrophenol
(40) in 25% yield, which was methylated using methyl iodide and
potassium carbonate in refluxing acetone providing 2-bromo-
6-nitroanisole (41) in 76% yield (the Scheme).40 Suzuki coupling of
compound 41 with 3-carboxyphenyl boronic acid with Pd(PPh3)4
and 2 M sodium carbonate in refluxing dioxane gave 20-methoxy-
30-nitrobiphenyl-3-carboxylic acid (42) in 47% yield as a tan
powder. Demethylation using 48% HBr (aq) in refluxing acetic acid
resulted in a 79% yield of 20-hydroxy-30-nitrobiphenyl-3-carboxylic
acid (43). The nitro group of compound 43 was reduced via catalytic
hydrogenation at 50 psi at room temperature over Pd/C in
mixed ethanol/3 M aq NaOH solution to give 30-amino-20-hydroxybiphenyl-
3-carboxylic acid (44) in quantitative yield. The
intermediate 1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1Hpyrazol-
5-one (47) was prepared by condensing of 3,4-dimethylphenyl-
hydrazine 45 with ethyl acetoacetate 46 with sodium
acetate in refluxing acetic acid in 76% yield. Treatment of (44) with
sodium nitrite in 1 M HCl at 5??C, followed by condensation with
1-(3,4-dimethylphenyl)-3-methyl-2,5-dihydro-1H-pyrazol-5-one
(47) at a constant pH of 7¨C8 via the addition of sodium bicarbonate
and ethanol afforded eltrombopag in 32% yield. Finally, eltrombopag
was treated with hydroxyl ethylamine to give eltrombopag
olamine (VIII). 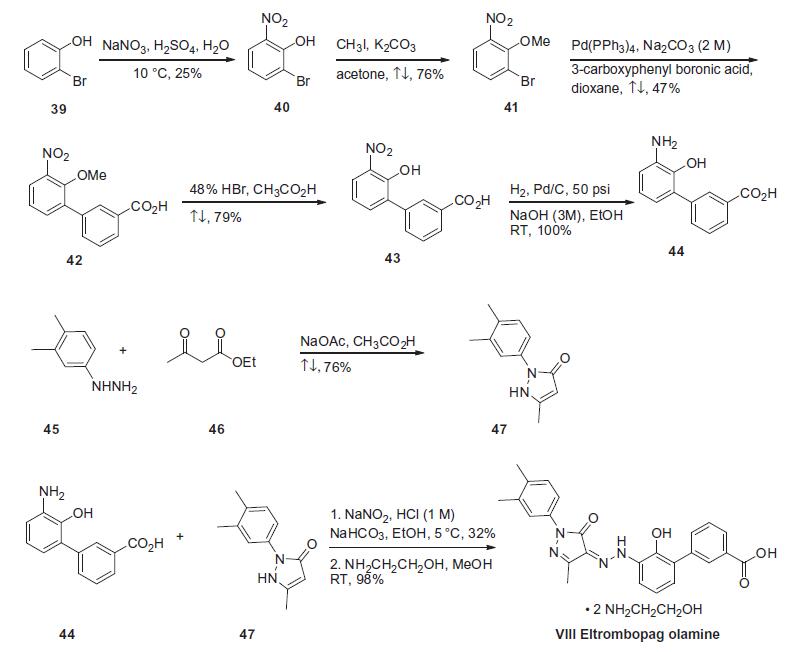
|
| | Unii-4U07F515lg Preparation Products And Raw materials |
|