|
| | DICHLORPHENAMIDE Basic information |
| Product Name: | DICHLORPHENAMIDE | | Synonyms: | 4,5-dichlorobenzene-1,3-disulphonamide;Dichlorfenamide;Antidrasi;CB 8000;LABOTEST-BB LT00771920;DICHLORPHENAMIDE;4,5-DICHLOROBENZENE-1,3-DISULFONAMIDE;Dalanide | | CAS: | 120-97-8 | | MF: | C6H6Cl2N2O4S2 | | MW: | 305.16 | | EINECS: | 204-440-6 | | Product Categories: | Inhibitors | | Mol File: | 120-97-8.mol |  |
| | DICHLORPHENAMIDE Chemical Properties |
| | DICHLORPHENAMIDE Usage And Synthesis |
| Chemical Properties | Off-White Solid | | Originator | Daranide,MSD ,US,1958 | | Uses | Like the drugs described above, dichlorphenamide is used for secreting excess water in
cases of elevated intraocular pressure, and it is used for treating wide-angle and secondary
glaucoma as well as in cases where it is necessary to lower intraocular pressure before surgical
intervention in severe wide-angle glaucoma. | | Uses | Diclofenamide is a potent carbonic anhydrase inhibitor. Diclofenamide may provide a therapeutic strategy in the inhibition of the human cytosolic isoforms I and II and transmembrane tumor-associated isoenzymes IX and XII. Diclofenamide has been shown to be is effective in the prevention of episodic weakness in both hypokalemic periodic paralysis (HypoPP) and potassium-sensitive periodic paralysis (PSPP). | | Uses | Diclofenamide is a potent carbonic anhydrase inhibitor. Diclofenamide may provide a therapeutic strategy in the inhibition of the human cytosolic isoforms I and II and transmembrane tumor-associated i
soenzymes IX and XII. Diclofenamide has been shown to be is effective in the prevention of episodic weakness in both hypokalemic periodic paralysis (HypoPP) and potassium-sensitive periodic paralysis
(PSPP). | | Definition | ChEBI: A sulfonamide that is benzene-1,3-disulfonamide in which the hydrogens at positions 4 and 5 are substituted by chlorine. An oral carbonic anhydrase inhibitor, it partially suppresses the secretion (inflow) of aqueous humor in the eye and so reduces intraoc
lar pressure. It is used for the treatment of glaucoma. | | Manufacturing Process | In a 2 liter round-bottomed flask equipped with stirrer and dropping funnel is
placed 1,585 grams (880 cc; 13.6 mols) of chlorosulfonic acid. To this is
added dropwise with stirring during 5 hours 218 grams (1.7 mols) of ochlorophenol. The mixture is allowed to stand 1 hour at room temperature
and then is heated 1 hour on a steam bath. The mixture is then poured on
ice.
A product consisting largely of 5-chloro-4-hydroxybenzene-1,3-disulfonyl
chloride separates as a gum which solidifies on standing for about 1 hour. The
solid product is collected on a Buchner funnel, washed with water and
thoroughly dried in air at room temperature.
A mixture of this crude product (approximately 302 grams, 0.92 mol) and 480
grams (2.3 mols) of phosphorus pentachloride is heated for 1 hour at 120°-
140°C in a 2 liter round-bottomed flask. The resulting clear solution is poured
on ice. 4,5-Dichlorobenzene-1,3-disulfonyl chloride separates immediately as a
solid. It is collected by filtration and washed with water. While still moist, it is
added in portions during about 20 minutes to 1 liter of concentrated ammonia
water contained in a 3 liter beaker surrounded by a cold water bath. The
reaction mixture is then allowed to stand for 1 hour without cooling after
which it is heated on a steam bath for about 30 minutes while air is bubbled
through it, in order to remove some of the excess ammonia. It is then
filtered, acidified with concentrated hydrochloric acid and chilled.
The product separates as a gum from which the supernatant liquid is
decanted, and the gum is triturated with 250 cc of water in order to induce
crystallization. The crude product thus obtained is recrystallized from 3,200 cc of boiling water and then from 40% aqueous isopropyl alcohol yielding 4,5-
dichlorobenzene-1,3-disulfonamide as a white solid, MP 228.5° to 229.0°C. of boiling water and then from 40% aqueous isopropyl alcohol yielding 4,5-
dichlorobenzene-1,3-disulfonamide as a white solid, MP 228.5° to 229.0°C. | | Brand name | Daranide (Merck). | | Therapeutic Function | Carbonic anhydrase inhibitor, Antiglaucoma
| | Clinical Use | Dichlorphenamide is a disulfonamide derivative that
shares the same pharmacological properties。The dose of dichlorphenamide is 25 to 100 mg one to three times
a day. | | Synthesis | Dichlorphenamide, 4,5-dichlorbenzol-1,3-disulfonamide (21.2.6), is
made in a relatively simple way from 2-chlorophenol. 2-Chlorophenol undergoes sulfochlorination
by chlorosulfonic acid, forming 4-hydroxy-5-chlorobenzol-1,3-
disulfonylchloride (21.2.4). The hydroxyl group is replaced by a chlorine atom using phosphorous
pentachloride, giving 4,5-dichlorobenzol-1,3-disulfonylchoride (21.2.5), the reaction
of which with ammonia gives the desired dichlorphenamide (21.2.6). 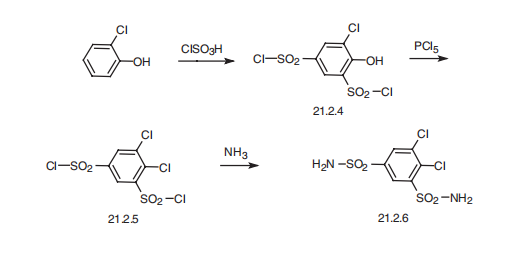
| | Veterinary Drugs and Treatments | Dichlorphenamide is used for the medical treatment of glaucoma.
Because of availability issues and toxic effects associated with systemic
therapy, human (and many veterinary) ophthalmologists are
using topical carbonic anhydrase inhibitors (e.g., dorzolamide or
brinzolamide) in place of acetazolamide, dichlorphenamide or methazolamide. |
| | DICHLORPHENAMIDE Preparation Products And Raw materials |
|



