|
| | TERT-BUTYL ISOCYANIDE Basic information |
| | TERT-BUTYL ISOCYANIDE Chemical Properties |
| Melting point | 148-150 °C | | Boiling point | 91 °C(lit.) | | density | 0.735 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.376(lit.) | | Fp | -2 °C | | storage temp. | Inert atmosphere,2-8°C | | solubility | Soluble in organic solvents such as ethanol, methanol, ether, toluene and dichloromethane. | | form | liquid | | color | colorless | | Specific Gravity | 0.735 | | BRN | 1903156 | | Exposure limits | NIOSH: IDLH 25 mg/m3 | | Stability: | store cold | | CAS DataBase Reference | 7188-38-7(CAS DataBase Reference) |
| Hazard Codes | F,T | | Risk Statements | 11-23-2017/11/23 | | Safety Statements | 16-45 | | RIDADR | UN 1993 3/PG 2 | | WGK Germany | 3 | | RTECS | EQ7102500 | | HazardClass | 3 | | PackingGroup | II | | HS Code | 29299090 |
| | TERT-BUTYL ISOCYANIDE Usage And Synthesis |
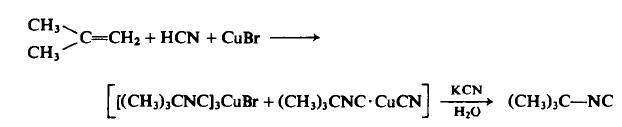
| Chemical Properties | clear colorless liquid | | Uses | tert-Butyl isocyanide is used in a tin-free procedure for alkyl radical reactions in the presence of a free-radical initiator. It is a useful intermediate in multicomponent reactions. It is also used in the synthesis of coumarines, 4H-chromenes, isoxazolines and to trap 2-cyclopropylidene-1,3-diones. | | Preparation | CAUTION: Use a well-ventilated hood and observe all precautions when using hydrogen cyanide.
To a 1-1, stainless steel Hoke pressure cylinder in a well-ventilated hood is added 196 gm (3.5 moles) of isobutene, 95 gm (3.5 moles) of hydrogen cyanide, and 100 gm (0.7 mole) or cuprous bromide. The cylinder is closed, shaken for 15 hr at 70°C, cooled to room temperature, and vented cautiously in the hood, and the viscous residue is poured slowly into aqueous potassium cyanide (260 gm in 100 ml of water) to decompose the cuprous complex, liberating an organic layer. The organic layer is separated, dried, and distilled under reduced pressure to afford 97 gm, b.p. 60-63°C (314 mm Hg), ng 1.3749, IR 2143 s, 1472 m, 1368 m, 1230 m, 1208 m, 855 w c m - 1; yield, 33% based on starting isobutylene; 16% based on CuBr.
| | Preparation | Phosgene (1.0 kg, 10.1 mol) was delivered through a wide tube into a stirred solution of N-t-butylformamide (1.01 kg, 10.0 mol) in triethylamine (1.30 kg) and o-dichlorobenzene (7.0 L) in a flask fitted with a reflux condenser charged with a freezing mixture of ice and salt (-20 C°). Water was added, the layers were separated, and the non-aqueous layer was dried over anhydrous potassium carbonate or magnesium sulfate and fractionated; bp 90–92 C°/750 mmHg; yield: 681 g (82%). | | General Description | Carbon dative bond formation by tert-butyl isocyanide on the germanium (100) surface has been reported. It also forms complexes with metals and inserts into metal-carbon bonds to form iminoacyls. |
| | TERT-BUTYL ISOCYANIDE Preparation Products And Raw materials |
|



