|
| | 4'-Bromo-3'-methylacetophenone Basic information |
| | 4'-Bromo-3'-methylacetophenone Chemical Properties |
| Melting point | 31-32 °C | | Boiling point | 157-159 °C(Press: 19 Torr) | | density | 1.388±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | fused solid | | color | Pale lemon |
| | 4'-Bromo-3'-methylacetophenone Usage And Synthesis |
| Uses | 4'-Bromo-3'-methylacetophenone is a versatile building block that is used in the synthesis of many complex compounds. It can be used as a reactant, reagent, or speciality chemical. | | Synthesis |
A solution of 4-bromo-3-methylbenzaldehyde (4 g, 20 mmol) in dry tetrahydrofuran (40 mL) was cooled to 0??C under nitrogen atmosphere, and then methyl magnesium bromide (20 mL, 1N in tetrahydrofuran) was added dropwise. The ice bath was removed, and the mixture was stirred for 2 hours. Ammonium chloride aqueous (40 mL) was added and the mixture was extracted with dichloromethane (20 mL * 3). The organic phase was dried by sodium sulphate, filtered and concentrated to give a residue. The residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate = 5:1) to give 1-(4-bromo-3-methylphenyl)ethanol. Colorless oil, yield 4.0 g, 93%. Pyridinium chlorochromate (48 g, 223 mmol) was added to a solution of 1-(4-bromo-3-methylphenyl)ethanol (31.9 g, 148 mmol) in dichloromethane (800 mL). The mixture was stirred at room temperature for 2 hours. The mixture was concentrated to give a residue. The residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate = 25:1) to give 4'-Bromo-3'-methylacetophenone. Yield 27.3 g, 87%.
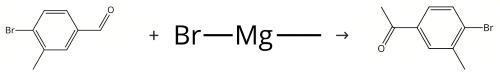 Figure Synthesis of 4'-Bromo-3'-methylacetophenone
Figure Synthesis of 4'-Bromo-3'-methylacetophenone
|
| | 4'-Bromo-3'-methylacetophenone Preparation Products And Raw materials |
|


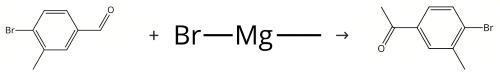 Figure Synthesis of 4'-Bromo-3'-methylacetophenone
Figure Synthesis of 4'-Bromo-3'-methylacetophenone
