|
| | Clozapine Chemical Properties |
| Melting point | 182-185°C | | Boiling point | 482.71°C (rough estimate) | | density | 1.1327 (rough estimate) | | refractive index | 1.6110 (estimate) | | Fp | 9℃ | | storage temp. | 2-8°C | | solubility | ethanol: 1 mg/mL | | pka | 3.70, 7.60(at 25℃) | | form | powder | | color | pale yellow | | Merck | 14,2423 | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. | | CAS DataBase Reference | 5786-21-0(CAS DataBase Reference) | | NIST Chemistry Reference | Clozapine(5786-21-0) |
| | Clozapine Usage And Synthesis |
| General description | Clozapine appears as pale yellow crystalline powder. It is odorless, tasteless with a molecular weight of 326.83 and a melting point 183~184 °C. It is soluble in chloroform, ethanol but insoluble in water. It is withdrawn from market because of causing neutropenia in 1976. Later studies have shown that this product has a good efficacy in treating schizophrenia, and thus had re-entered into market in 1989, 1990, in Sweden and the United States, respectively. This product is a non-classical antipsychotic which can selectively block DA receptors of the mesolimbic system but has a small effect on the nigrostriatal DA receptors. It has a relatively weak blocking effect on D2 receptor, but a strong blocking effect on D1 receptors strong while also has some antagonist effect on D4 receptor and 5-HT2 receptor. Thereby, it has strong anti-psychotic effects. Moreover EPS is less common and does not cause stiff reaction. Owing to the direct inhibition effect on the ascending reticular activating system of the brains, it has a potent sedative and hypnotic effects which can well applied for treating schizophrenia, hallucinations, delusions and youth-based effect. In addition to its good efficacy on treating positive symptoms like agitation, hallucinations, and paranoia, it also has a good efficacy on the negative symptoms. Since this product has a different mechanism of action on blocking DA receptors with traditional antipsychotics, thus this drug may also be effective in treating some classic antipsychotic disease which fails be treated with traditional drugs. It can also be used to alleviate mania and severe sleep disorders. In addition, it also has an anti-cholinergic effect. NE has sympathetic blocking effect, muscle relaxing effect and anti-histamine effect.
| | Pharmacological effects | 1. Antipsychotic effects: Clozapine has good efficacy on treating various types of acute and chronic psychiatric disease. It can alleviate symptoms like excitement, agitation, hallucinations, delusion, thought disorder, depersonalization, and strange movements. The mechanism may be associated with its blocking effect on the dopamine receptor of mesolimbic system, but does not affect the dopamine receptors in the striatum, so this product rarely produces extrapyramidal reactions.
2. Sedative and hypnotic effects: This product can inhibit the spontaneous activity when applied in low-dose. High dose can increase automatic activity. Clozapine can directly inhibit the brain ascending reticular activating system.
3. Anti-cholinergic, anti-histamine and anti-adrenergic effects: The product has a strong anti-acetylcholine effect, and also has suppressing effect on histamine, norepinephrine and epinephrine.
| | Pharmacodynamics | This product is a kind of major tranquilizer which has well therapeutic effect on treating acute and chronic schizophrenia. Its mechanism of action is probably playing a pharmacological effect on central dopamine nerve. It can selectively block dopamine receptors in the limbic system with small effect on nigrostriatal dopamine receptor, so it has a strong anti-psychotic effect. The extrapyramidal side effects are rare. Its function also has no dependence on the activity of adenylate cyclase. | | Pharmacokinetics | Its absorption through oral administration happens quickly and completely. There is no food effect on the rate and extent of absorption. It is widely distributed to various organizations after rapid absorption and there are large individual differences in bioavailability with an average deviation of about 50% to 60%. It also has hepatic first-pass effect. The plasma concentration reaches peak 3.2 hours (1-4 hours) after taking drugs and has an elimination half-life (t1/2β) about 9 hours. The apparent volume of distribution (Vd) is 4.04~13.78L/kg. It has a high tissue binding rate. After being metabolized by the liver, 80% of it is in the form of metabolites presented in urine and feces. Its major metabolites include N-demethylated clozapine, and the N-oxidant of clozapine. Under the same dose and a constant body weight, serum drug concentrations of female patients are significantly higher than that of male patients. Smoking can accelerate the metabolism of this product. Drug metabolism and renal clearance are significantly lower in the elderly. This product can be secreted from the milk, and can penetrate through the blood-brain barrier.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| | Uses | 1. It is used for treating acute, chronic mania. It can be used to stabilize the emotion of human. It has good efficacy on treating symptoms like, vagrancy, sleep disorder, sadness, panic, tiredness, easy to be ill, sudden violence, and anger.
2. It can be used for treating crazy disease due to a variety of reasons such as losing temper, aggressive, emotional instability, anxiety, tension, and manic, etc.
3. It can be used for treating mental retardation-caused behavioral disorders such as linguistic disorder, poor presentation ability, poor thought and mental ability, lack of abstraction, generalization ability, low perception, narrow perception range, being difficult to distinguish small differences in body shape, size and color, lack of concentration, narrow attention range, poor memory, slow memorizing and reproducing inaccurately, na?ve behavior, immaturity, emotional instability, lack of self-control and impulsiveness, frequently being timid, withdrawn, shy, asymmetrical shape, uncoordinated movements, poor flexibility, performance, or hyperactivity, destruction, aggression.
4. It can be used for treating drug or alcohol-dependence withdrawal syndrome such as acute tremors, agitation, being easy to get startled, and nausea, vomiting and sweating. Upon drinking, the symptoms disappeared immediately, otherwise last for several days. There is sometimes even a short delusions, hallucinations and visual distortion, lisp. Finally, the patient may have seizures or delirium tremens.
5. Can be used for treating insomnia. It is applicable to the various manifestations of insomnia, such as dreaminess and being easy to wake up, heart palpitations, forgetfulness, and tiredness.
| | Synthetic route | Use 2,5-dichloro-nitrobenzene (2) and anthranilic acid (3) to react in DMF to generate 2-(4-chloro-2-nitro) dimethylbenzene (4), dimethylbenzene undergoes hydrazine hydrate/ferric chloride catalytic reduction to produce 2-(2-amino-4-chlorophenyl) dimethylbenzene (5), dimethylbenzene goes through cyclization reaction with the catalysis of polyphosphoric acid to obtain 8-chloro-5,10-dihydro-11H-dibenzo [b, e] [1,4]-dinitrogen-11-one (6). Product in step 6 is condensed together with N-methylpiperazine in the effect of titanium tetrachloride to get the final product.

Figure 1 the synthesis route of clozapine
| | Precautions | 1. Induction of the decrease of neutropenia or its development into agranulocytosis is the most serious side effects of this product. So do not take drugs which causes leukopenia simultaneously while taking clozapine, especially avoid taking the traditional antipsychotic or antidepressant drugs or long-acting antipsychotics. Leukocyte count should be performed before treatment in order to understand the patient to return to normal polymorphonuclear leukocytes index. After the start of treatment, weekly leukocyte count should be performed for 18 consecutive weeks. Then the test should be done at least once a month. Do the same things during long-term medication. At each time of consultation, remind the patients to seek medical treatment immediately when infection or fever occurs. When there is an infection or WBC is less than 3.5 × 109/L or be much lower compared to the initial measured index, the white blood cell count should be reviewed immediately. If the absolute value of leukocyte reduced to 3 × 109/L or less and/or neutrophil polymorphonuclear leukocytes have an absolute value being less than 1.5 × 109/L, should be immediately discontinued.
2. For patients with a history of seizures or cardiovascular, renal, hepatic insufficiency, apply a lower initial dose and should increase the dose more gradually.
3. For patients with non-serious liver disease, clinical monitoring and regularly check of liver function is necessary.
4. Remind the patient that this product has the role of triggering drowsiness. So do not drive and operate machinery while taking the drug.
5. It has interaction with the central sedative and antipsychotic drugs, so be cautious when taking medicine.
6. Lactating women should not be breastfeeding upon medication.
7. Disable who are allergic to clozapine.
8. Patients with neutropenia, agranulocytosis or an apparent history of blood disease; Be cautious for patients of alcohol and drug-induced psychosis, poisoning and coma, severe heart, liver, kidney diseases, angle-closure glaucoma, and prostatic hypertrophy-caused urinary tract disease.
| | Side effects | Common adverse reactions include salivation (significantly during sleeping), nausea, vomiting, and abdominal distension. Occasionally fever, neutropenia, and a long-term drug addiction occur.
| | Contraindications | Children under 16 years of age, patients of angle-closure glaucoma, prostatic hyperplasia, spastic disorders or with a history of epilepsy and severe cardiovascular disease should take with caution. Disable it for patients with significantly inhibited central nervous system and with a history of myelosuppression or abnormal blood cells.
| | Chemical Properties | Light yellow crystalline powder with melting point 183-184 °C. It is easily soluble in chloroform, soluble in ethanol, almost insoluble in water. It is odorless, tasteless.
| | Uses | 1. The product has not only a strong anti-psychotic effect, but also a calming effect as a novel broad-spectrum psychiatric drug which is suitable for treating acute and chronic schizophrenia. It has a good efficacy on treating paranoid hallucinations, youth-based diseases. In the angle of alleviating the symptoms, it is quite effective in treating agitation, hallucinations, delusions, contacts, and apastia.
2. Used as antipsychotics
| | Production methods | 2-amino-4-chloro-diphenylamine-2'-carboxylic acid (4''-methyl) piperazine reacts with phosphorus oxychloride to get clozapine.
| | Description | Clozapine (5786-21-0) is a dopamine D4?and D2?receptor antagonist. High affinity for the cloned rat dopamine D4?receptor (Ki?< 20 nM).1?Atypical neuroleptic agent.2?Antagonist at 5HT2A, 5HT2C, 5HT3, 5HT6 and 5HT7 receptors.3,4 | | Chemical Properties | Yellow Crystalline Solid | | Originator | Leponex,Wander,W. Germany,1974 | | Uses | depigmentor | | Uses | An antipsychotic | | Uses | Clozapine is a neuroleptic, which expresses antipsychotic and sedative action. It does not
cause general depression and extrapyramidal disorders. It is used for severe and chronic forms of schizophrenia, maniacal conditions, manic-depressive psychosis, psychomotor
excitement, and various other psychotic conditions. | | Definition | ChEBI: A benzodiazepine that is 5H-dibenzo[b,e][1,4]diazepine substituted by a chloro group at position 8 and a 4-methylpiperazin-1-yl group at position 11. It is a second generation antipsychotic used in the treatment of psychiatr
c disorders like schizophrenia. | | Manufacturing Process | 7.4 g of 2-amino-4-chlorodiphenylamine-2'-carboxylic acid (4"-methyl)
piperazide and 35 ml of phosphoroxychloride are heated for 3 hours under
reflux in the presence of 1.4 ml of N,N-dimethylaniline. Upon concentration of
the reaction mixture in vacuo as far as possible, the residue is distributed
between benzene and ammonia/ice water. The benzene solution is extracted
with dilute acetic acid. The acid extract is clarified with charcoal and treated
with concentrated ammonia water to precipitate the alkaline substance, which
is dissolved in ether. The ethereal solution is washed with water and dried
over sodium sulfate. The residue obtained yields, after recrystallization from
ether/petroleum ether 2.9 g (41% of the theoretical yield) of 8-chloro-11-(4-
methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine in the form of yellow
grains of melting point 182° to 184°C (from acetone/petroleum ether). | | Brand name | Clozaril (Novartis); Fazaclo (Avanir);Leponox. | | Therapeutic Function | Tranquilizer | | World Health Organization (WHO) | Clozapine, a tricyclic neuroleptic, was introduced in 1972 for the
treatment of psychosis. In 1975 its use was associated with cases of
agranulocytosis, particularly in Finland. These cases, which included several
fatalities, resulted in the withdrawal of the drug in some countries. However,
clozapine remains available in at least 30 countries, in some cases only on special
request, for the treatment of severe psychotic disorders unresponsive to other
neuroleptics provided that close monitoring of the blood count is feasible. In 1989,
it was introduced in the United States for the treatment of severe schizophrenia.
Lately, the use of clozapine in the United Kingdom has been associated with
convulsions.
(Reference: (WHODIB) WHO Drug Information Bulletin, 2: 10, , 1977) | | General Description | The dibenzodiazepine derivative is clozapine(Clozaril). It is not a potent antipsychotic on a milligrambasis (note the orientation of the N-methyl piperazino grouprelative to the chlorine atom). In addition to their moderatepotencies at DA receptors (mainly D4), clozapine interactwith varying affinities at several other classes of receptors(α1 and α2 adrenergic, 5-HT1A, 5-HT2A, 5-HT2C, muscariniccholinergic, histamine H1, and others). It is effective againstboth positive and negative symptoms of schizophrenia andhas a low tendency to produce EPS. Clozapine has proved effectiveeven in chronically ill patients who respond poorly tostandard neuroleptics. However, there are legal restrictionson its use because of a relatively high frequency of agranulocytosis.As a rule, two other antipsychotics are tried beforerecourse to therapy with clozapine. Clozapine is metabolized preferentially by CYP3A4 into demethylated, hydroxylated,and N-oxide derivatives that are excreted in urine and feces.Elimination half-life averages about 12 hours. Other clozapine-like atypical antipsychotics may lack a 2-Cl substituenton the aromatic ring (e.g., olanzapine and quetiapine). | | General Description | Clozapine, 8-chloro-11-(4-methyl-1-piperazinyl)5H-dibenzo[b,e] [1,4] diazepine (Clozaril), is a yellowcrystalline powder that is only slightly soluble in water. Withthe introduction of clozapine, a different pharmacological antipsychotics.106 Unlike typical antipsychotics, clozapine is largelydevoid of EPS. The lack of EPS with this compound waspostulated to be caused by its preferential binding tomesolimbic rather than striatal DA receptors.Furthermore,clozapine was shown to exhibit potent affinity for 5-HT2Areceptors.
DMCZ shows partial agonism at D2 and D3 receptors andexhibits a distinctly different pharmacological profile comparedwith clozapine and other atypical antipsychotics.Unlike clozapine, which is a potent M1 muscarinic antagonist,DMCZ is a potent M1 agonist. Agonism at muscarinicreceptors has been proposed to be useful for impaired cognitionin schizophrenia. Burnstein et al.found thatDMCZ acted as a partial agonist at DA D2 and D3 receptors.These investigators suggested that the low incidence of EPSassociated with clozapine may be caused by the partial agonismof DMCZ at D2 and D3 receptors. Thus, DMCZ maybe of interest as an atypical antipsychotic with an improvedside effect profile compared with clozapine. | | Biological Activity | Atypical antipsychotic drug, with a much lower tendency to cause extrapyramidal side effects than conventional neuroleptics. Displays a broad range of pharmacological actions; the antipsychotic effects are thought to be mediated principally by 5-HT 2A/2C and dopamine receptor blockade (K i values are 21, 170, 170, 230 and 330 nM for D 4 , D 3 , D 1 , D 2 and D 5 receptors respectively). | | Biochem/physiol Actions | Atypical antipsychotic compound. Selective antagonist for D4-dopamine receptor. Antagonist at 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, and 5-HT7 serotonin receptors. | | Clinical Use | Although clozapine demonstrates a favorable pharmacologicalprofile compared with typical antipsychotics, its useis restricted by a relatively high incidence of agranulocytosis.The exact mechanism for the cause of agranulocytosishas not been confirmed, but a highly reactive nitrenium ionthat is formed by the action of hepatic enzymes appears tobe involved.The mean elimination half-life of clozapine following a single 75-mg dose is 8 hours. Because of severaladverse effects, clozapine is only used in refractory casesof schizophrenia. Individuals with a history of seizures orpredisposed to seizures should be cautioned when takingclozapine. Similar to other atypical antipsychotic agents,clozapine causes an increased risk of mortality in elderly individualswith dementia-related psychoses. | | Side effects | A serious drawback to the use of clozapine, however, is the potentially fatal agranulocytosis that
is reported to occur in 1 to 2% of unmonitored patients, necessitating weekly white blood cell counts for at
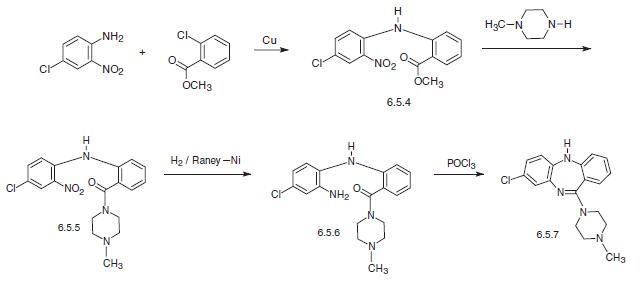
least the first 6 months of pharmacotherapy. | | Synthesis | Clozapine, 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e] [1,4]diazepine (6.5.7) is synthesized by two methods. According to the first, 4-chloro-2-nitroaniline in the presence of copper filings is acylated by the o-chlorobenzoic acid methyl ester, forming the corresponding diphenylamine (6.5.4). By reacting this with N-methyl piperazine, the ester group in the resulting polyfunctional diphenylamine is transformed into the amide (6.5.5). The nitro group in the resulting 4-chloro-2- nitro-2′-carb-(N′-methyl piperazino)amide (6.5.5) is further reduced into an amine group by hydrogen in the presence of Raney nickel. Reacting the resulting product (6.5.6) with phosphorous oxychloride yields in heterocyclization into the desired dibenzodiazepine, clozapine (6.5.7).
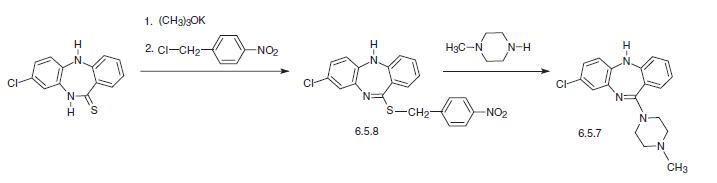
The other way of synthesis of clozapine is from 8-chloro-10,11-dihydro-5H-dibenzo[b,e]1, 4-diazepin-11-thione, which is alkylated at the sulfur atom of the dibenzodiazepine ring by 4-nitrobenzylchloride in the presence of potassium tert-butoxide, giving N-methyl derivative (6.5.8). Reaction of this with N-methylpiperazine gives the desired clozapine (6.5.7).
| | Drug interactions | Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong
the QT interval; increased risk of arrhythmias with
flecainide.
Antibacterials: concentration possibly increased
by erythromycin (possible increased risk
of convulsions); concentration increased by
ciprofloxacin; concentration possibly reduced
by rifampicin; avoid with chloramphenicol and
sulphonamides (increased risk agranulocytosis).
Antidepressants: concentration possibly increased
by citalopram, fluoxetine, fluvoxamine, paroxetine,
sertraline and venlafaxine (increased risk of toxicity);
possibly increased CNS effects of MAOIs; possibly
increased antimuscarinic effects with tricyclics;
increased concentration of tricyclics.
Antiepileptics: antagonises anticonvulsant effect;
metabolism accelerated by carbamazepine, phenytoin
and possibly phenobarbital; avoid with drugs known
to cause agranulocytosis; concentration possibly
increased or decreased by valproate.
Antimalarials: avoid with artemether/lumefantrine.
Antipsychotics: avoid with depot formulations
(cannot be withdrawn quickly if neutropenia occurs);
possible increased risk of ventricular arrhythmias
with risperidone - avoid.
Antivirals: concentration increased by ritonavir -
avoid; increased risk of ventricular arrhythmias with
saquinavir - avoid.
Anxiolytics and hypnotics: increased sedative effects;
adverse reports with clozapine and benzodiazepines.
Atomoxetine: increased risk of ventricular
arrhythmias.
Cytotoxics: increased risk of agranulocytosis - avoid;
increased risk of ventricular arrhythmias with arsenic
trioxide.
Lithium: increased risk of extrapyramidal side effects
and possibly neurotoxicity.
Penicillamine: increased risk of agranulocytosis -
avoid.
Ulcer-healing drugs: effects possibly enhanced
by cimetidine; concentration possibly reduced by
omeprazole. | | Metabolism | Clozapine is orally active and metabolized mainly by CYP3A4 to
inactive desmethyl, hydroxyl, and N-oxide derivatives, with a half-life of approximately 12 hours. Clozapine has relatively low affinity for brain dopamine D1 and D2 receptors (moderate affinity for D4) in comparison to its affinity
at adrenergic α1 and α2, histamine H1, muscarinic M1 and serotonin 5-HT2A receptors. | | storage | Room temperature | | References | 1) Seeman and Van Tol (1994),?Dopamine receptor pharmacology;? Trends Pharmacol. Sci.,?15?264
2) Ellenbroek?et al. (1991),?The involvement of dopamine D1 and D2 receptors in the effects of the classical neuroleptic haloperidol and the atypical neuroleptic clozapine;? Eur. J. Pharmacol.,?196?103
3) Canton?et al.?(1990),?Binding of the typical and atypical antipsychotics to 5-HT1C and 5-HT2 sites: clozapine potently interacts with 5-HT1C sites;? Eur. J. Pharmacol.,?191?93
4) Kuoppamaki?et al.?(1993),?Clozapine and N-desmethylclozapine are potent 5-HT1C receptor antagonists;? Eur. J. Pharmacol., 245?179 |
| | Clozapine Preparation Products And Raw materials |
|





