|
| | Aluminum trifluoromethanesulfonate Basic information |
| Product Name: | Aluminum trifluoromethanesulfonate | | Synonyms: | ALUMINIUM TRIFLATE;ALUMINIUM TRIFLUOROMETHANESULFONATE;ALUMINIUM TRIFLUOROMETHANESULPHONATE;ALUMINUM TRIFLATE;ALUMINUM TRIFLUOROMETHANESULFONATE;Aluminumtrifluoromethanesulfonate,99%(Aluminumtriflate);Aluminium triflate~Trifluoromethanesulphonic acid aluminium salt;ALUMINUM TRIFLUOROMETHANESULFONATEALUMINUM TRIFLUOROMETHANESULFONATEALUMINUM TRIFLUOROMETHANESULFONATEALUMINUM TRIFLUOROMETHANESULFONATE | | CAS: | 74974-61-1 | | MF: | CH4AlF3O3S | | MW: | 180.08 | | EINECS: | | | Product Categories: | metal triflate compounds;triflate | | Mol File: | 74974-61-1.mol |  |
| | Aluminum trifluoromethanesulfonate Chemical Properties |
| Melting point | 300 °C(lit.) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | soluble in ether, acetone, acetonitrile, and diglyme; poorly soluble in nitromethane; sparingly soluble in SO2 and SO2 ClF. | | form | Powder | | color | White | | Water Solubility | Insoluble in water. | | Hydrolytic Sensitivity | 6: forms irreversible hydrate | | Sensitive | Hygroscopic | | Stability: | hygroscopic | | CAS DataBase Reference | 74974-61-1(CAS DataBase Reference) |
| Hazard Codes | C,Xi | | Risk Statements | 34-36/37/38 | | Safety Statements | 26-36/37/39-45-36 | | RIDADR | UN 3261 8/PG 3 | | WGK Germany | 3 | | Hazard Note | Corrosive | | TSCA | No | | HazardClass | 8 | | PackingGroup | III | | HS Code | 29049020 |
| | Aluminum trifluoromethanesulfonate Usage And Synthesis |
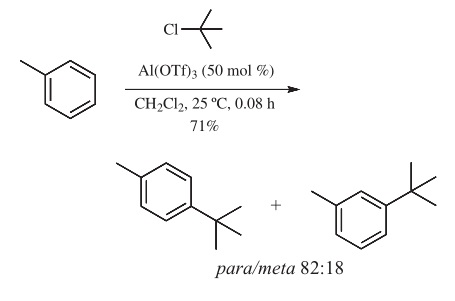
| Chemical Properties | White powder | | Uses | Lewis acid used as a catalyst for Friedel–Crafts, ketalization, nucleophilic substitution, hydroalkoxylation, methoxycarbonyla- tion, rearrangement, and epoxide ring-opening reactions. | | Uses | Aluminum trifluoromethanesulfonate is used in pharmaceutical intermediates. | | Preparation | Can be prepared from aluminum trichloride and tribromide by heating with triflic acid in anhydrous conditions. Preparations from triethylaluminum, aluminum carbide, and aluminum isopropoxide have also been described. | | Reactions | Friedel-Crafts Reactions.
Aluminum trifluoromethanesulfonate has been used for the Friedel-Crafts alkylation reaction of toluene with isopropyl and tert-butyl chlorides (eq 1), and for theacylationofbenzeneandtoluenewithacetylandbenzoylchlo- rides in low to moderate yields. Intramolecular Friedel-Crafts acylation of an aromatic compound with Meldrum’s acid has also been reported using catalytic amounts of Al(OTf) 3 . Acylation of 2-methoxynaphthalene with acetic anhydride has been reported using Al(OTf) 3 and lithium perchlorate as an additive to afford the corresponding 6-acetylated adduct in 83% yield. Effective acylation of arenes with carboxylic acids has also been disclosed using polystyrene-supported Al(OTf)3.
|
| | Aluminum trifluoromethanesulfonate Preparation Products And Raw materials |
|