|
| | DRONEDARONE HYDROCHLORIDE Basic information |
| | DRONEDARONE HYDROCHLORIDE Chemical Properties |
| Melting point | NA (low-melting) | | storage temp. | 2-8°C | | solubility | DMSO: soluble15mg/mL, clear | | form | powder | | color | white to off-white | | Merck | 14,3449 | | Stability: | Hygroscopic | | InChIKey | DWKVCQXJYURSIQ-UHFFFAOYSA-N |
| RIDADR | UN 3077 9 / PGIII | | WGK Germany | 3 | | HS Code | 2935904000 |
| | DRONEDARONE HYDROCHLORIDE Usage And Synthesis |
| Chemical Properties | Pale Yellow Solid | | Uses | Dronedarone Hydrochloride can be used for the treatment of atrial fibrillation and atrial flutter in patients who have suffered cardiac arrhythmias.
| | Uses | Cardiovascular Drugs | | Uses | Dronedarone Hydrochloride is a therapy for the treatment of patients with paroxysmal and persistent atrial fibrillation or atrial flutter.
| | Biological Activity | dronedarone hcl is an amiodarone analogue which has been shown an effective and promising treatment for atrial fibrillation (af) [1]. | | Biochem/physiol Actions | Dronedarone is a Class III antiarrhythmic and a multi-channel blocker for atrial fibrillation. It blocks potassium, sodium, and calcium channels and also exhibits antiadrenergic properties. | | Clinical Use | Dronedarone hydrochloride (also known as SR33589 and marketed
as Multaq) is a drug developed by Sanofi-Aventis for cardiac
arrhythmias (irregular heartbeat) that was approved by the FDA in
July 2009. Dronedarone is used for the treatment of atrial fibrillation
and atrial flutter in patients whose hearts have either returned
to normal rhythm or who undergo drug therapy or
electroshock treatment to maintain normal cardio rhythm. Dronedarone
is less lipophilic than amiodarone, exhibits a much smaller
volume of distribution and a half-life of 24 h, this stands in contrast
to competitor amiodarone’s half-life of several weeks. As
a result of these pharmacokinetic characteristics, dronedarone
dosing may be less complicated than amiodarone. | | Synthesis | The synthesis
of dronedarone relies on the preparation of the benzofuran core
34, of which three main routes have been reported, but two possess
obvious overlap and are considered more process-amenable.
Starting from methyl 2-(2-formylphenoxy)hexanoate (32), this aldehyde can either be nitrated, then saponified or saponified
and then nitrated to procure nitroacid 33 (the Scheme). The benzofuran
ring is then secured through the use of acetic anhydride and
base in the presence of DMF at elevated temperature. The key benzofuran
34 can be produced by either route in 62% yield on gramscale
by this method. Friedel¨CCrafts acylation involving anisoyl
chloride and tin tetrachloride constructed the diaryl ketone 35.
Cleavage of the methyl ether through the use of aluminum trichloride
in refluxing DCE provided phenol 36. Alkylation of phenol 36
with aminoalkyl chloride 37 gave ether 38. Subsequent reduction
of the nitro group via catalytic hydrogenation and sulfonylation
of the resulting amine provided dronedarone (VII) which was isolated
as its HCl salt. 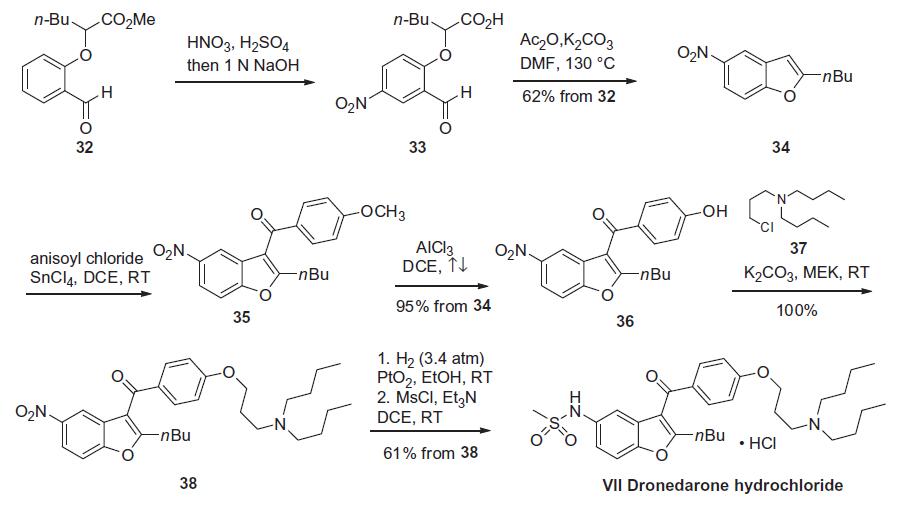
| | in vitro | dronedarone has been demonstrated to inhibit muscarinic acetylcholine receptor-operated k+ current ik(ach) induced by carbachol or gtp-gamma-s with ic50 values of 10nm and <100nm, respectively, in cells isolated from guinea pig atria. notably, dronedarone was 100-fold potent and selective over amiodarone in inhibiting ik(ach) [1]. | | in vivo | dronedarone has shown to block arterial thrombus formation, decrease platelet aggregation and reduce plasminogen activator inhibitor-1 (pai1) expression in c57bl/6 mice [2]. | | references | [1] guillemare e1, marion a, nisato d, gautier p. inhibitory effects of dronedarone on muscarinic k+ current in guinea pig atrial cells. j cardiovasc pharmacol. 2000 dec;36(6):802-5.
[2] breitenstein a1, sluka sh, akhmedov a, stivala s, steffel j, camici gg, riem hh, beer hj, studt jd, duru f, luscher tf, tanner fc. dronedarone reduces arterial thrombus formation. basic res cardiol. 2012 nov;107(6):302. |
| | DRONEDARONE HYDROCHLORIDE Preparation Products And Raw materials |
|