|
| | Vardenafil hydrochloride Basic information |
| Product Name: | Vardenafil hydrochloride | | Synonyms: | 1-[[3-(1,4-dihydro-5-methyl-4-oxo-7-propylimidazo[5,1-f][1,2,4]triazin-2-yl)-4-ethoxyphenyl]sulfonyl]-4-ethyl-piperazine dihydrochloride;2-[2-Ethoxy-5-(4-ethylpiperazin-1-yl)sulfonylphenyl]-5-methyl-7-propyl-1H-imidazo[5,1-f][1,2,4]triazin-4-one hydrochloride;vardenafil hydrochloride;VARDENAFIL HCL;Vardenafil Hydrochloride Trihydrate 224785-90-4 /;Levitra,Valdenafil.HCl;1-[[3-(1,4-Dihydro-5-methyl-4-oxo-7-propylimidazo[5,1-f][1,2,4]triazin-2-yl)-4-ethoxyphenyl]sulfonyl]-4-ethyl-piperazine;Nuviva | | CAS: | 224785-91-5 | | MF: | C23H33ClN6O4S | | MW: | 525.07 | | EINECS: | 606-590-1 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Vardenafil;API;224785-91-5 | | Mol File: | 224785-91-5.mol |  |
| | Vardenafil hydrochloride Chemical Properties |
| Melting point | 214-216°C | | storage temp. | Store at -20°C | | solubility | DMSO: 100 mg/mL (190.45 mM); Water: ≥ 100 mg/mL (190.45 mM) | | form | Solid | | color | White to Off-White Cyrstalline | | InChIKey | XCMULUAPJXCOHI-UHFFFAOYSA-N | | SMILES | C(C1=NC(C)=C2C(N=C(C3C=C(S(N4CCN(CC)CC4)(=O)=O)C=CC=3OCC)NN12)=O)CC.Cl |
| | Vardenafil hydrochloride Usage And Synthesis |
| Description | Vardenafil was the second agent to be marketed and had the advantage that its onset time was not
reduced by taking the medication on a full stomach . It is 30 times more potent
as an inhibitor of PDE5 (mean IC50, 3.9 nM) than sildenafil and 10 times more potent than tadalafil,
with a greater selectivity (>1,000 times) for human PDE5 than for human PDE2, PDE3, and PDE4
and moderate selectivity (>80 times) for PDE1. The PDE inhibitory selectivity
and both the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Vardenafil specifically
inhibited the hydrolysis of cGMP by PDE5, with an IC50 of 0.7 nM (sildenafil 6.6 nM). The IC50 of
vardenafil for PDE1 was 180 nM, for PDE6 11 nM, and for PDE2, PDE3 and PDE4 more than 1,000
nM. | | Chemical Properties | White to Off-White Cyrstalline Solid | | Uses | vardenafil hydrochloride is a selective phsphodiesterase type 5 (pde5) inhibitor that is used as a urological agent in the treatment of erectile dysfunction. | | General Description | Vardenafil, 4-[2-ethoxy-5-(4-ethylpiperazin-1-yl)sulfonyl-phenyl]-9-methyl-7-propyl-3,5,6,8-tetrazabicyclo[4.3.0]nona-3,7,9-trien-2-one(Levitra), was the second PDE5 introduced in the U.S. market.The metabolism of vardenafil is primarily by CYP3A4.As such, concomitant use of CYP3A4 inhibitors such as ritonavir,indinavir, ketoconazole, as well as moderate CYP3Ainhibitors such as erythromycin typically results in significantincreases of plasma levels of vardenafil. | | Synthesis | The synthesis started with 2-
hydroxybenzonitrile. 2-Hydroxybenzonitrile (181) was
alkylated with ethyl bromide to give 2-ethoxybenzonitrile in
97% yield as a liquid which was subsequently treated with
AlMeClNH2, prepared from AlMe3 and NH4Cl, to give
corresponding 2-ethoxybenzamidine (182) in 76% yield as a
solid. Compound 182 was treated with hydrazine hydrate in
ethanol to give hydrazide 183, which was used in the next
step without isolation. Dakin-West reaction of 2-
butyrylaminopropionic acid (184) with ethyl oxalyl chloride
(185) in the presence of DMAP in refluxing pyridine/THF to
give corresponding |á-oxoamino-acid ester 186 which was
also used for next step without isolation. Hydrazide 183 was
condensed with ester 186 in refluxing ethanol to give
triazinone 187 intermediate which was then cyclized to the
final core structure, imidazo[5,1-f]triazin-4-one, using
POCl3 to give 188 in 28% yield from 183. Compound 188
was sulfonylated with chlorosulfonic acid to give sulfonyl
chloride 189 in 91% yield. Finally, 189 was condensed with N-ethylpiperazine (190) in dichloromethane to give
vardenafil (XXIII) in 66% yield. 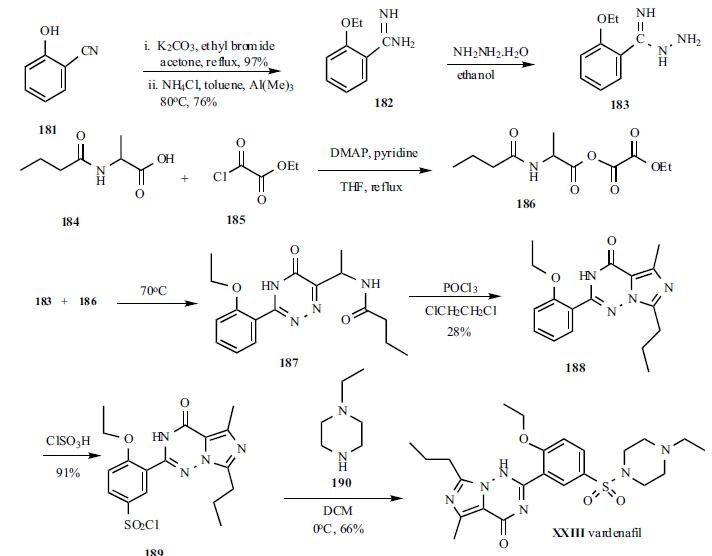
| | Enzyme inhibitor | Vardenafil also is rapidly absorbed and peaks in concentration (9.05 μg/mL after a 10-mg dose) after 0.9 hours, displaying a half-life of
4 to 5 hours. The absorption rate of both sildenafil and vardenafil are reduced when taken with a high-fat diet. The drug also is
metabolized by hepatic CYP3A4, and a potential for drug–drug interaction with inhibitors or enhancers of CYP3A4 exists. Biochemical
studies demonstrate a significant increase in selectivity of vardenafil over sildenafil for PDE5 versus PDE6. Whether this translates
into a significant improvement in side effects must await studies in a greater population of patients. |
| | Vardenafil hydrochloride Preparation Products And Raw materials |
|