|
| | Trimethylamine Basic information |
| | Trimethylamine Chemical Properties |
| Melting point | -117 °C (lit.) | | Boiling point | 3-4 °C (lit.) | | density | 0.63 g/mL at 20 °C (lit.) | | vapor density | 2.09 (vs air) | | vapor pressure | 430 mm Hg ( 25 °C) | | refractive index | n20/D 1.357 | | FEMA | 3241 | TRIMETHYLAMINE | | Fp | 38 °F | | storage temp. | Store at +5°C to +30°C. | | solubility | very soluble in water, slightly soluble in alcohol, ether, benzene, toluene, xylene, ethylbenzene, chloroform maximum allowable concentration: TLV 10 p.p.m. (24 mg/m3) and STEL of 15 p.p.m. (36 mg/m3) (ACGIH 1986) | | form | Liquid | | pka | pKb (25°): 4.13 | | color | Colorless | | PH | a strong base (pH 9.8) | | Odor | smelling like rotting fish, rotting eggs, garbage, or urine. | | Odor Threshold | 0.000032ppm | | Odor Type | fishy | | explosive limit | 11.6% | | Water Solubility | Soluble In Water, 8.9e+005 mg/L. | | FreezingPoint | -117.1℃ | | Sensitive | Hygroscopic | | Merck | 14,9710 | | JECFA Number | 1610 | | BRN | 956566 | | Exposure limits | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin)
OSHA: TWA 200 ppm(590 mg/m3)
NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) | | Stability: | Stable. Incompatible with a wide variety of materials, including bases, acids, oxidizing agents, brass, zinc, magnesium, aluminium, mercury, mercury oxides, acid chlorides, acid anhydrides. Hygroscopic. Highly flammable. Readily forms explosive mixtures with air. | | InChIKey | GETQZCLCWQTVFV-UHFFFAOYSA-N | | LogP | 0.06 | | CAS DataBase Reference | 75-50-3(CAS DataBase Reference) | | EPA Substance Registry System | Trimethylamine (75-50-3) |
| | Trimethylamine Usage And Synthesis |
| Chemical Properties | Trimethylamine is compressed gas or liquid.
Flammable gas. Shipped as a compressed gas, it may be
present in an aqueous solution. It has a strong, fishy, ammoniacal
odor. The Odor Threshold is 0.00011-0.87 ppm.
Warning: The Odor Threshold range is so broad that odor
alone should not be used as a warning of potentially
hazardous exposures. | | Physical properties | Trimethylamine has a pungent, fishy, ammoniacal odor at low concentration.It's a colourless liquid with a boiling point around 3.5°C, compared with the higher melting point of 224-226°C for the more polar Me3NO, which presumably has dipole-dipole intermolecular forces.
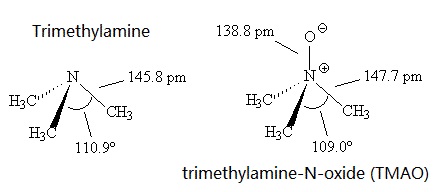
Trimethylamine is a base, like ammonia. Also like ammonia, it has a trigonal pyramidal structure. The C-N-C bond angle is 110.9°, compared with 107.2° in NH3, presumably due to greater repulsions between the methyl groups. This angle is reduced to 109.0° in Me3NO.
| | Occurrence | TMA is widely distributed in the environment as a normal constituent of animal and plant tissue and as a result of its formation during the decay of organic matter in plants, animals, fish, sewage and animal waste (Graedel 1978; Hippe et al 1977; Oremland et al 1982). The amine is formed primarily as the result of microbial degradation of the plant and animal constituents betaine and choline and from bacterial reduction of trimethylamine oxide, a common constituent of aquatic organisms. It also occurs naturally in a variety of foodstuffs and in tobacco smoke and these are the most likely sources of human exposure (HSDB 1988).
Numerous strains of bacteria isolated from various sources have been found capable of growing on TMA (HSDB 1988). Degradation products formed under anaerobic conditions include dimethylamine, formaldehyde, formate and C02, while under aerobic conditions, TMA is converted to dimethylamine, ammonia and methane. | | Uses | Trimethylamine is used as a warning agent for natural gas, a synthetic flavor (fish) ingredient, and in the synthesis of photochemicals, choline salts, flotation agents, dyes, pesticides, ion-exchange resins, cationic starches, and intense sweeteners (HSDB 2006).
Organic synthesis, especially of choline salts, warning agent for natural gas, manufacture of disinfectants, flotation agent, insect attractant, quaternary ammonium compounds, plastics.
| | Definition | ChEBI: Trimethylamine is a tertiary amine that is ammonia in which each hydrogen atom is substituted by an methyl group. It has a role as a human xenobiotic metabolite and an Escherichia coli metabolite. It is a tertiary amine and a member of methylamines. It is a conjugate base of a trimethylammonium. | | Preparation | Trimethylamine can be synthesized from paraformaldehyde and ammonium chloride, by the reaction of formic acid, formaldehyde, and ammonia, and by interaction of methanol and ammonia with a catalyst at high temperature. | | Production Methods | Trimethylamine (TMA) is produced by several methods: from the reaction of ammonia and methanol; from paraformaldehyde and ammonium chloride; by the action of formaldehyde and formic acid on ammonia; and by the interaction of methanol and ammonia over a catalyst at high temperature (Hawley 1981; HSDB 1988). TMA is sold as an aqueous solution or as a liquefied gas (Windholz et al 1983) in which the aqueous solution is available as 25, 30, and 40% and anhydrous as 99% minimum. The impurities consist of ammonia at no more than 0.2% by weight of solution and formaldehyde at no more than 0.3% by wt. of solution (Rick 1985). U.S. production was estimated to be approximately 15,322 tons in 1984 (HSDB 1988). | | Reactions | Trimethylamine (TMA) has been used in the preparation of poly[9,9′-bis(6′-N,N,N-trimethylammonium)hexyl)fluorene-co-alt-4,7-(2,1,3-benzothiadiazole) dibromide] (PFBT), a water-soluble, cationic conjugated polymer used in label-free DNA microarrays. It can also be used to prepare benzyltrimethylammonium chloride, which then reacts with sodium ethoxide to form benzyltrimethylammonium ethoxide.The adsorption of TMA on the gold surface of trimethylsilylated barium nitrate-gold/titanosilicate catalyst acts as a promoter for the propylene epoxidation with oxygen and hydrogen. | | Aroma threshold values | Detection: 0.3 to 0.8 ppb; recognition: 500 ppb | | General Description | A colorless gas with a fishlike odor at low concentrations changing to ammonia-like odor at higher concentrations. Shipped as a liquid under its own vapor pressure. Contact with the unconfined liquid can cause frostbite from evaporative cooling or chemical type burns. The gasis corrosive and dissolves in water to form flammable, corrosive solutions. Gas is an asphyxiate by the displacement of air. Produces toxic oxides of nitrogen during combustion. Prolonged exposure to heat can cause the containers to rupture violently and rocket. Long-term inhalation of low concentrations or short -term inhalation of high concentrations has adverse health effects. | | Air & Water Reactions | Highly flammable and easily ignited. Water soluble. | | Reactivity Profile | TRIMETHYLAMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. Contamination of an ethylene oxide tank with trimethylamine caused an explosion [BCISC Quart. Safety Summ., 1966, 37, 44]. | | Health Hazard | TMA is formed naturally from the biodegradation of plants, fish and animal products, and is ingested in foods such as fish, or from foods containing TMA precursors [e.g. trimethylamine oxide (TMAO), choline, and L-carnitine], which are metabolized to TMA by enterobacteria (Bain et al. 2005). Human exposure to TMA vapor has caused respiratory and eye irritation and corneal lesions. Effects in laboratory animals consisted of respiratory tract toxicity (gasping, labored breathing, lung lesions), eye lesions, neurotoxicity (apathy, splayed hind- or forelimbs, uncoordinated movements, convulsions, brain lesions), and some studies also found pathological changes of the liver, spleen, and kidneys. | | Safety Profile | Poison by intravenous
route. Moderately toxic by subcutaneous
and rectal routes. Mildly toxic by inhalation.
A very dangerous fire hazard when exposed
to heat or flame. Self-reactive. Moderately
explosive in the form of vapor when
exposed to heat or flame. Can react with
oxidizing materials. To fight fire, stop flow
of gas. Potentially explosive reaction with
bromine + heat, ethylene oxide,
triethynylaluminum. When heated to
decomposition it emits toxic fumes of NOx.
See also AMINES. | | Potential Exposure | Trimethylamine is used as a chemical
intermediate in organic synthesis of quaternary ammonium
com pounds; as an insect attractant; as a warning agent in
natural gas; flotation agent. | | Carcinogenicity | No studies were found that examined the carcinogenicity of Trimethylamine in humans. Because mechanisms have been proposed by which the known carcinogen N47 nitrosodimethylamine can be formed from Trimethylamine and TMAO (Bain 2005) in the presence of nitrosating agents, there is some concern about the neoplastic potential of Trimethylamine. Thus, the German exposure guidelines warn that co-exposure to Trimethylamine and nitrosating agents should be minimized (see Section 8.2.). However, a 2-year mouse and rat inhalation study with the related amine DMA, which can also potentially form N-nitrosodimethylamine, showed no tumor formation despite severe chronic nasal lesions (CIIT 1990). | | Purification Methods | Dry triethylamine by passing the gas through a tower filled with solid KOH. Water and impurities containing labile hydrogen were removed by treatment with freshly sublimed, ground, P2O5. It has been refluxed with acetic anhydride, and then distilled through a tube packed with HgO and BaO. [Comyns J Chem Soc 1557 1955.] For more extensive purification, trimethylamine is converted to the hydrochloride, crystallised (see below), and regenerated by treating the hydrochloride with excess aqueous 50% KOH, the gas is passed through a CaSO4 column into a steel cylinder containing sodium ribbon. After 1-2 days, the cylinder is cooled to -78o and hydrogen and air are removed by pumping. [Day & Felsing J Am Chem Soc 72 1698 1950.] Me3N has been distlled from trap-to-trap and degassed by freeze-pump-thaw [Halpern et al. J Am Chem Soc 108 3907 1986]. It is commercially supplied in a pressure tin. [Beilstein 4 H 43, 4 I 322, 4 II 553, 4 III 99, 4 IV 134.] | | Incompatibilities | A medium strong base. Violent reaction
with strong oxidizers (such as chlorine, bromine, fluorine),
ethylene oxide; nitrosating agents, for example, nitrites,
sodium nitrite, nitrous gases, nitrous acid) capable of
releasing carcinogenic nitrosamines.); keep away from mercury,
strong acids. Corrosive to many metals, for example,
zinc, brass, aluminum, copper, tin, and their alloys. | | Waste Disposal | Return refillable compressed
gas cylinders to supplier. Nonrefillable cylinders should be
disposed of in accordance with local, state and federal regulations.
Allow remaining gas to vent slowly into atmosphere
in an unconfined area or exhaust hood. Refillabletype
cylinders should be returned to original supplier with
any valve caps and outlet plugs secured and valve protection
caps in place. |
| | Trimethylamine Preparation Products And Raw materials |
|



