| Indications and Usage | 5-Aza-2’-deoxycytidine, also known as decitabine or 2'-deoxy-5-azacytidine, is a cell cycle S phase-specific drug used to treat myelodysplastic syndromes (MDS.)
|
| Mechanisms of Action | Demethylating agents can regulate gene expression by activating tumor suppressor genes and enhancing the differentiation of genes, which help treat MDS. 5-Aza-2’-deoxycytidine is a natural andenosine analogue of 2′-deoxycytidine, known to the be strongest methylation specific inhibitor of DNA. After phosphorylation it can inhibit DNA methyltransferase, reducing DNA methylation, leading to demethylation of tumor cells, which can restore the normal functioning of genes, thus inhibiting tumor cell proliferation and preventing the occurrence of drug resistance. 5-Aza-2’-deoxycytidine inhibits DNA methylation in vitro, but does not affect its synthesis. Moreover, non-proliferating cells are not sensitive to it. 5-Aza-2’-deoxycytidine has anti-tumor activity and is characterized by a dual mechanism based on dose differences. At high concentrations it is cytoxic, while at low concentrations it is demethlyating.
|
| Side Effects |
- Neutropenia, thrombocytopenia, anemia, vomiting, fatigue, fever, cough, nausea, constipation, diarrhea, hyperglycemia, and heat induced neutrophil.
- Large doses may cause neurotoxicity, manifested as lethargy, aphasia, hemiplegia, etc., which disappears after stopping.
|
| Category | toxic substance |
| Toxicity grading | high
|
| Acute toxicity | Intravenous – mouse LD50: 22 mg/kg; peritoneal – mouse LD50: 190 mg/kg
|
| Flammability Hazardous characteristics | Combustible; produces toxic nitrogen oxide fumes.
|
| Storage characteristics | Ventilated, cold, and dry; store away from raw food materials.
|
| Fire extinguishing agent | dry powder, foam, sand, carbon dioxide, water mist.
|
| Description | Decitabine, 5-aza-2’-deoxycytidine, has been launched for the treatment of
myelodysplastic syndromes (MDS). MDS are a set of hematologic disorders
affecting the bone marrow that result in ineffective formation and development
of blood cells. Furthermore, patients with MDS have a high risk of progressing to
acute myeloid leukemia (AML). Traditional treatments include blood transfusions,
hematopoietic growth factors, and prophylactic antibiotics, but these
measures merely improve the quality of life with questionable effects on disease
modification. While stem-cell transplantation is an aggressive, potentially curative
approach, the advanced age or the other complicating health issues of most
patients preclude them from considering this option. Recent advances in the
underlying etiology of MDS, however, have led to the development of a new
class of compounds known as ‘‘demethylating agents’’. Decitabine follows the
successful introduction of the first DNA methyltransferase inhibitor azacitidine. |
| Chemical Properties | white crystalline powder |
| Originator | Pharmachemie (Netherlands) |
| Uses | 5-Aza-2′-deoxycytidine has been used as a demethylating agent in breast cancer cell line, chromatin, DNA and promoter region of p16 gene. |
| Uses | Used as cancer treatment, in particular to inhibit the growth of pancreatic endocrine tumor cell lines. |
| Uses | Decitabine is a potent inhibitor of DNA methylation with IC50 of 438 nM and 4.38 nM in HL-60 and KG1a cells, respectively |
| Definition | ChEBI: 5-aza-2'-deoxycytidine is a 2'-deoxyribonucleoside. |
| Brand name | Dacogen (Millot Laboratories, France). |
| General Description | Fine white crystalline powder. Used as a drug. |
| Air & Water Reactions | Probably light and air sensitive. Water soluble. Decomposes in aqueous solution at a rate that depends on pH: at pH 7 the drug is more stable than at pH 9, but is less stable than at pH 6. At pH 7 and 99°F, approximately 7% conversion occurs in 1 hour . |
| Reactivity Profile | 5-Aza-2'-deoxycytidine is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. |
| Fire Hazard | Flash point data for 5-Aza-2'-deoxycytidine are not available. 5-Aza-2'-deoxycytidine is probably combustible. |
| Biological Activity | Cytosine analog that once incorporated into DNA acts as a suicide substrate for DNA methyltransferase. Inhibits DNA methyltransferase and results in DNA hypomethylation and activation of silent genes. Chemotherapeutic agent; suppresses growth of human tumor cell lines. Demethylates differentiation-related genes; reverses embryonic stem cell differentiation. |
| Biochem/physiol Actions | Primary TargetDNA methyltransferase inhibitor |
| Clinical Use | Antineoplastic antimetabolite agent:
Treatment of acute myeloid leukaemia |
| Safety Profile | Poison by intravenous route.Human mutation data reported. When heated todecomposition it emits toxic fumes of NOx. |
| Synthesis | Silylated 5-aza-cytosine (28) was condensed with 9-
fluorenylmethoxycarbonyl (Fmoc) protected 2-deoxy-1-chlororibose
(27) with tin chloride (IV) in dichloroethane (Scheme
4). The coupled product 29 was de-protected with excess
triethylamine in dry pyridine to give decitabine (IV) in 36%
yield after separation from its corresponding |áisomer. 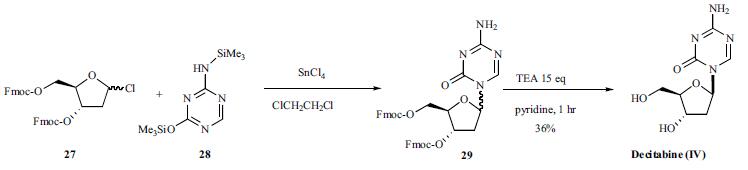
|
| target | Telomerase | DNA Methyltransferase | p21 |
| Drug interactions | Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis. |
| Metabolism | The exact route of metabolism and elimination is
unknown but thought to be through deamination
by cytidine deaminase in the liver, kidney, intestinal
epithelium and blood to form inactive metabolites. |
| storage | +4°C |
| References | 1) Bender et al. (1998), Inhibition of DNA methylation by 5-aza-2′-deoxycytidine suppresses the growth of human tumor cell lines; Cancer Res., 58 95
2) El-Serafi et al. (2011), Epigenetic modifiers influence lineage commitment of human bone marrow stromal cells: Differential effects of 5-aza-deoxycytidine and trichostatin A; Differentiation, 81 35 |