| | Oxandrolone Chemical Properties |
| Melting point | 235-238°C | | alpha | D25 -23° (c = approx 1% in chloroform) | | Boiling point | 387.04°C (rough estimate) | | density | 1.0829 (rough estimate) | | refractive index | 1.4200 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO: soluble2mg/mL (clear solution; warmed) | | pka | 15.15±0.60(Predicted) | | form | powder | | color | white to beige | | optical activity | [α]/D -19 to -26°, c = 1 (CDCl3) | | Merck | 6921 | | InChIKey | QSLJIVKCVHQPLV-PEMPUTJUSA-N | | CAS DataBase Reference | 53-39-4(CAS DataBase Reference) | | NIST Chemistry Reference | Oxandrolone(53-39-4) |
| | Oxandrolone Usage And Synthesis |
| testosterone | Oxandrolone is an anabolic steroid, a testosterone analog with ten times the anabolic activity but one-tenth of the androgenic activity of testosterone. Research into its use in burn injury for preventing loss or restoring muscle mass has been going on since the mid-1990s. Demling found that patients in the rehabilitation phase on an exercise programme, oxan- drolone and a high protein diet gained more body weight than those on an exercise programme and normal or high protein diet. Oxandrolone was effective in older adults, over 60 years, as well as in younger adults. This is important in light of the difficulties older adults face due to loss of lean body mass.
In 2000 Demling used oxandrolone (20 mg/ day) in the acute phase on burn injury, beginning when patients reached a minimum of 75% of their energy and protein requirements. Twenty patients were studied in a double-blind randomised controlled trial. Patients on oxandrolone had an improved net nitrogen balance, decreased weight loss, faster healing of donor sites and a shorter length of stay. Oxandrolone did not decrease metabolic rate and there were no side effects. The use of oxandralone is being considered in burns in the UK.
| | Pharmacokinetics | Oxandrolone is administered orally and is rapidly absorbed. Oxandrolone is highly bound (9–97%) to plasma proteins and has a bioavailability of approximately 97%. Hepatic metabolism of oxandrolone is markedly slower than that of testosterone or other androgens due to the modification of ring A (lack of a 4-ene function) and 17alfa-alkylation. The elimination half-life for oxandrolone is approximately 9.4 h, and peak plasma concentrations are higher than methyltestosterone. Oxandrolone is excreted primarily in the urine as the unchanged parent drug (approximately 28%) and unconjugated product. | | Uses | The use of oxandrolone, an analog of testosterone possessing only 5% of testosterone's virilizing androgenic effects, enhances anabolism of muscle protein by improving the efficiency of protein synthesis in severely burned children. Oxandrolone administration decreases loss of body weight and improves healing of the donor site wound. In a large clinical trial by our group, 0.1 mg/kg oxandrolone administered twice daily reduced length of the acute hospitalization, sustained LBM, and improved liver protein synthesis. Severely burned pediatric patients receiving oxandrolone for 1 year experienced improved growth, decreased cardiac work, and improved muscle strength. Oxandrolone treatment also improved lung function at rest and during exercise in this patient population. These improvements were maintained for up to 4 years after treatment had ended. The benefits of oxandrolone administration after burn injury were further enhanced when the treatment period was increased from 1 to 2 years. | | Indications and Dosages | It is important to note that when treating wasting conditions with oxandrolone (or any other androgen), proper nutrition is essential. Oxandrolone is indicated for the treatment of wasting syndromes associated with chronic diseases and lack of nutrition. Oxandrolone has also been suggested for use in the treatment of AIDS-related wasting syndrome, Duchenne muscular dystrophy, growth failure, and Turner’s syndrome. However, these uses have not been approved by the FDA. Oxandrolone is indicated for treating wasting conditions (cachexia, resulting from chronic disease and/or infection, severe trauma, prolonged glucocorticoid treatment, or extensive surgery), thereby promoting weight gain and increased protein synthesis. Furthermore, treatment would be indicated within those individuals who fail to maintain normal body weight. The usual recommended oral dosage in adults is 2.5 mg, taken 2-4 times daily (5-10 mg/day) over a period of 2-4 weeks. Depending on how the patient responds, the treatment may be repeated as needed. If necessary, the dosage may be increased up to 20 mg/day. However, the patient’s response to treatment will determine the dose and duration of treatment. In children, the recommended oral dose is 0.1 mg/kg (0.045 mg/pound) body weight/day over a period of 2–4 weeks. However, the dosage should not exceed the adult dosage. | | Side effects | No significant side effects have been reported in boys treated with oxandrolone for CDGD. Although oxandrolone has significantly fewer androgenic effects than testosterone, mild virilization has been reported in girls taking oxandrolone, including clitoromegaly. This is less of a concern at lower doses. There are also reports of a delay in breast development that improves upon higher estrogen dosing. Hepatic dysfunction has been reported with oxandrolone treatment, manifested by alterations in HDL cholesterol, and thus monitoring of lipids is suggested. | | overdose | No symptoms or signs associated with overdosage have been reported. It is possible that sodium and water retention may occur.
The oral LD50 of oxandrolone in mice and dogs is greater than 5,000 mg/kg. No specific antidote is known, but gastric lavage may be used.
| | Related Articles | Oxandrolone is a man-made steroid, similar to the naturally occurring steroid testosterone. Oxandrolone is an "anabolic" steroid that promotes the growth of muscle tissue.
https://www.drugs.com/mtm/oxandrolone.html
Oxandrolone is a synthetic, orally active anabolic-androgenic steroid. Oxandrolones interact with androgen receptors in target tissues.
https://drugs.ncats.io/substance/7H6TM3CT4L
Oxandrolone is an anabolic steroid that has been used in catabolic situations such as hepatitis and AIDS patients and can be administered orally, thus eliminating one source of stress for children and families.
https://www.sciencedirect.com/topics/neuroscience/oxandrolone
Oxandrin (oxandrolone) is indicated as adjunctive therapy to promote weight gain after weight loss following extensive surgery, chronic infections, or severe trauma, and in some patients who without definite pathophysiologic reasons fail to gain or to maintain normal weight, to offset the protein catabolism associated with prolonged administration of corticosteroids, and for the relief of the bone pain frequently accompanying osteoporosis (See DOSAGE AND ADMINISTRATION).
https://www.rxlist.com/oxandrin-drug.htm#indications
| | Description | Oxandrolone is a synthetic anabolic-androgenic steroid. It is a 17 alpha-methylated version of dihydrotestosterone (DHT). It can be used for the treatment of many kinds of disorders such as idiopathic short stature, body mass loss due to catabolic illness, severe burns, trauma and hereditary angioedema as well as turner syndrome. Oxandrolone is especially effective in the treatment of severe burns without causing obvious side effects. It acts as an agonist of the androgen receptor, which modulates related gene expression to increase protein synthesis, further boosting muscle growth and increasing body mass as well as bone mineral density. However, it should be noted that its androgenic effect is less than its anabolic effect.
| | Chemical Properties | white Solid. Soluble in chloroform, slightly soluble in ethanol, acetone, slightly soluble in ether, very slightly soluble in water. | | Originator | Anavar,Searle,US,1964 | | Uses | androgenic anabolic steroid;reverses catabolic tissue processes; promotes buildup of protein; increases erythropoietin production | | Uses | Oxandrolone is used for the same indications as nandrolone. It is a synthetic, anabolic steroid. Used to promote muscle growth and combat involuntary weight loss. It has also been used to treat cases of osteoporosis. | | Definition | ChEBI: Oxandrolone is a 3-oxo steroid, an oxa-steroid, a 17beta-hydroxy steroid and an anabolic androgenic steroid. It has a role as an androgen and an anabolic agent. | | Brand name | Oxandrin (Savient). | | Therapeutic Function | Androgen | | General Description | Oxandrolone, 17β-hydroxy-17-methyl-2-oxaandrostan-3-one, is approved to aid in the promotionof weight gain after weight loss following surgery,chronic infections, or severe trauma and to offset protein catabolismassociated with long-term corticosteroid use.Oxandrolone is also used to relieve bone pain accompanyingosteoporosis. It has been used to treat alcoholic hepatitisand HIV wasting syndrome. | | Biochem/physiol Actions | Oxandrolone is a synthetic anabolic steroid. It is is a non-aromatizable androgen with no estrogenic effects and with mild androgenic activity. Clinical uses of oxandrolone include to promote weight gain after weight loss following extensive surgery or chronic infections or trauma, to offset the protein catabolism associated with prolonged administration of corticosteroids, to relieve bone pain frequently accompanying osteoporosis, and to treat Turner′s syndrome in girls. | | Safety Profile | Moderately toxic by ingestion andintraperitoneal routes. Experimental reproductive effects.When heated to decomposition it emits acrid smoke andfumes. | | Synthesis | Oxandrolone, 17|?-hydroxy-17|á-methyl-2-oxa-5-androstan-3-one (29.3.10),
is made by oxidation of the C1¨CC2 double bond of 17|?-hydroxy-17|á-methyl-1-androsten-
3-one by a mixture of lead tetraacetate and osmium tetroxide with an opening of the A ring
of the steroid system, which forms an aldehyde acid (29.3.9). Upon reducing the aldehyde
group with sodium borohydride, intramolecular cyclization takes place, directly forming a
lactone (29.3.10), which is the desired oxandrolone. 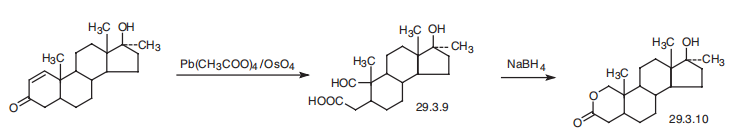
| | References | Li, H., et al. "The efficacy and safety of oxandrolone treatment for patients with severe burns: A systematic review and meta-analysis. " Burns Journal of the International Society for Burn Injuries 42.4(2016): 717.
Hart, D. W., et al. "Anabolic effects of oxandrolone after severe burn." Annals of Surgery 233.4(2001): 556-564.
Sheffieldmoore, M, et al. "Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. " Journal of Clinical Endocrinology & Metabolism 84.8(1999): 2705-11.
https://en.wikipedia.org/wiki/Oxandrolone
|
| | Oxandrolone Preparation Products And Raw materials |
|




