|
| | Promazine Basic information |
| Product Name: | Promazine | | Synonyms: | PHENOTHIAZINE,10-(3-(DIMETHYLAMINO)PROPYL)-;N,N-Dimethyl-3-(10H-phenothiazin-10-yl)propan-1-amine;dimethyl(3-phenothiazin-10-ylpropyl)amine;A-145;RP-3276;Wy-1094;Promazine;N,N-dimethyl-3-phenothiazin-10-yl-propan-1-amine | | CAS: | 58-40-2 | | MF: | C17H20N2S | | MW: | 284.4191 | | EINECS: | 2003820 | | Product Categories: | | | Mol File: | 58-40-2.mol |  |
| | Promazine Chemical Properties |
| Melting point | 25°C | | Boiling point | bp0.3 203-210° | | density | 1.1256 (rough estimate) | | refractive index | 1.6000 (estimate) | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | pKa 9.4(H2O,t =24±1) (Uncertain) | | color | Off-White to Pale Beige | | Water Solubility | 14.22mg/L(24 ºC) | | Stability: | Hygroscopic | | NIST Chemistry Reference | Promazine(58-40-2) |
| | Promazine Usage And Synthesis |
| Uses | ntipsychotic. | | Uses | Promazine is an antipsychotic and a tranquilizer. | | Uses | In psychiatric practice, promazine is used in minor cases of psychomotor excitement in
schizophrenics, in paranoid and manic-depressive conditions, for neurosis, alcoholic
psychosis, and others. It is sometimes used in anesthesiological practice. | | Definition | ChEBI: A phenothiazine deriative in which the phenothiazine tricycle has a 3-(dimethylaminopropyl) group at the N-10 position. | | Brand name | Sparine (Baxter Healthcare); Sparine (Wyeth). | | General Description | Promazine, 10-[3-(dimethylamino) propyl-(phenothiazine monohydrochloride (Sparine), was introducedinto antipsychotic therapy after its 2-chloro-substitutedrelative. The 2H-substituent vis-à-vis the 2Clsubstituent gives a milligram potency decrease as an antipsychotic,as encompassed in Gordon’s rule. Tendency toEPS is also lessened, which may be significant, especially ifit is decreased less than antipsychotic potency. | | Synthesis | Promazine, 10-(3-dimethylaminopropyl)phenothiazine (6.1.1), is prepared
by the alkylation of phenothiazine with 3-dimethylaminopropylchloride in the presence of
sodium amide [1¨C3]. 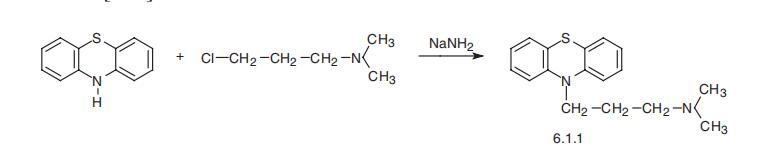
|
| | Promazine Preparation Products And Raw materials |
|