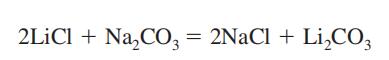| Description | Lithium carbonate (molecular structure is Li2CO3, English name is lithium carbonate) as a colorless monoclinic crystal or white powder. Density is 2.11. Melting point is 618 ℃. Without deliquescence, it is stable in the air. Low solubility in water, the solubility decreases with increasing temperature. Solubility in cold water is greater than hot water. It is Soluble in dilute acid, insoluble in alcohol and acetone. Carbon dioxide is introduced into the aqueous suspension of lithium carbonate, lithium carbonate is converted to lithium acid carbonate and dissolved. If the solution of lithium acid carbonate is heated and then it releases carbon dioxide and precipitates lithium carbonate. The nature of the lithium carbonate may be used to remove impurities from lithium carbonate. Since lithium ion has a strong polarizability, thus thermal stability of lithium carbonate is worse than other alkali metal carbonate, when heated to above the melting point, it will decompose to produce carbon dioxide and lithium oxide.
 |
| Chemical Properties | Lithium carbonate is a white monoclinic crystalline solid. Typically for carbonates, lithium carbonate reacts with acids stronger than carbon dioxide or carbonic acid to yield the lithium salt of the acid and carbon dioxide. The reactions may be carried out in a solution, as an aqueous slurry, or, less effectively, with solid lithium carbonate.
Lithium carbonate exhibits a low water solubility for an alkali metal carbonate. The solubility decreases with increasing temperature. It is not hygroscopic and is generally stable when exposed to the atmosphere. In fact, it is the normal end compound encountered when many basic lithium compounds are exposed to the atmosphere. Lithium carbonate may be dissolved in water by conversion to the hydrogen carbonate. Releasing carbon dioxide by heating the solution of lithium hydrogen carbonate causes reprecipitation of the lithium carbonate.
|
| Uses | The lithium carbonate industry is a global high monopoly industry, the current production capacity is mainly concentrated in three foreign manufacturers of SQM, FMC, Chemetall and so on.
Industrial lithium carbonate is used in the manufacture of other lithium salts, such as lithium chloride and lithium bromide and so on. It also acts as lithium oxide materials in enamel, glass, pottery and porcelain enamel, and it is also added to the electrolytic cell for electrolysis of aluminum to increase the current efficiency and reduce the internal resistance of the cell and the bath temperature. In medicine, it is mainly used for the treatment of mania, can improve their emotional disorders for schizophrenia. It has the effect of elevating peripheral leukocytes; can be used for synthetic rubber, dyes, semiconductor and military defense industry and so on; for the production of lithium tantalate, lithium niobate and other acoustic grade single crystal, optical grade monocrystalline etc; for preparation of the acoustic grade single crystal.
Battery grade lithium carbonate is mainly used for the preparation of lithium cobalt oxide, lithium manganese oxide, ternary materials, lithium iron phosphate and other lithium ion battery cathode materials; used in a matrix modifier; as aneuroprotective effect of lithium carbonate in amyotrophic lateral sclerosis.
|
| Toxicity | Lithium carbonate has a significant stimulating effect, firstly has damage on the gastrointestinal tract, kidney and central nervous system. Toxicity order of lithium compounds is Li <LiCl <Li2CO3, maximum allowable concentration: Lithium condensation and fragmentation aerosol were 0.05 mg/m3 and 0.5 mg/m3.
Wear rubber gloves and protective masks when working, in order to protect the respiratory organs against dust.
|
| Preparation | Lithium carbonate is obtained as an intermediate product in recovery of lithium metal from its ore, spodumene (See Lithium). It is prepared by mixing a hot and concentrated solution of sodium carbonate with lithium chloride or sulfate solution.
Li2SO4+ Na2CO3→Li2CO3+ Na2SO4 |
| Reactions | Lithium carbonate reacts with dilute acids, liberating carbon dioxide:
Li2CO3+ HCl →LiCl + CO2+ H2O
Thermal decompostion yields lithium oxide and carbon dioxide:
Li2CO3 → Li2O + CO2
Reaction with lime produces lithium hydroxide:
Li2CO3+ Ca(OH)2→2LiOH + CaCO3
The carbonate reacts with molten aluminum fluoride converting to lithium fluoride:
3Li2CO3+ 2AlF3 → 6LiF + 3CO2+ Al2O3
It combines with carbon dioxide in aqueous slurry forming soluble bicarbonate, which decomposes to carbonate upon heating:
Li2CO3+ CO2+ H2O →2LiHCO3
The bicarbonate can not be separated in solid form. It exists only in solution when carbonate dissolves in water saturated with CO2under pressure.
|
| Chemical Properties | Lithium carbonate is a white hygroscopic powder. |
| Physical properties | White monoclinic crystals; refractive index 1.428; density 2.11 g/cm3; melts at 723°C; decomposes at 1,310°C; low solubility in water (1.54 g/100g) at 0°C; 1.32 g//100g at 20°C), solubility decrease with temperature (0.72g/100g at 100°C); insoluble in acetone and ethanol. |
| Uses | The most common lithium drug is lithium carbonate, which possesses antimania action. It
is presumed that lithium alters the transport of sodium ions in neurons, thus influencing
the intercellular contents of catecholamines, normalizing the mental state and not causing
general lethargy. It is used for mania conditions of various origins, preventative measures,
and for treating affective psychoses. |
| Uses | Lithium carbonate is used as a compound for producing metallic
lithium. Lithium carbonate is the result of treating the mineral spodumene with sulfuric
acid and then adding calcium carbonate. It is used as an antidepressant. |
| Uses | In the production of glazes on ceramic and electrical porcelain. |
| Preparation | Lithium carbonate is prepared by the precipitation of lithium
ion by carbonate ion from an aqueous solution. Still another process, which is carried out on a smaller
scale, is the reaction of a solution of lithium hydroxide with carbon dioxide gas. Lithium
carbonate precipitates and is recovered from the supernatant solution. |
| Definition | lithium carbonate: A white solid,Li2CO3; r.d. 2.11; m.p. 723°C; decomposesabove 1310°C. It is producedcommercially by treating the ore with sulphuric acid at 250°C andleaching the product to give a solutionof lithium sulphate. The carbonateis then obtained by precipitationwith sodium carbonate solution.Lithium carbonate is used in the preventionand treatment of manicdepressivedisorders. It is also usedindustrially in ceramic glazes. |
| Indications | Lithium inhibits thyroidal incorporation of I- into Tg, as
well as the secretion of thyroid hormones, but it does
not inhibit the activity of the Na+-I- symporter or the
accumulation of I- within the thyroid. Lithium offers no
particular advantage over drugs of the thionamide class
but may be employed for temporary control of thyrotoxicosis
in patients who are allergic to both thionamides
and iodide. |
| Brand name | Eskalith (GlaxoSmithKline); Lithane (Bayer); Lithobid
(JDS); Lithonate (Solvay Pharmaceuticals). |
| General Description | Lithiumcarbonate (Eskalith, Lithane) and lithium citrate(Cibalith-S) are the salts commercially available in theUnited States. |
| Reactivity Profile | A base. Decomposed by acids with the evolution of carbon dioxide. Fluorine burns fiercely on contact with Lithium carbonate. |
| Flammability and Explosibility | Nonflammable |
| Clinical Use | Treatment and prophylaxis of mania, manic
depressive illness, and recurrent depression
Aggressive or self-mutilating behaviour |
| Safety Profile | Human carcinogenic
data. Poison by intraperitoneal and
intravenous routes. Moderately toxic by
ingestion and subcutaneous routes. Human
systemic effects by ingestion: toxic
psychosis, tremors, changes in fluid intake,
muscle weakness, increased urine volume,
nausea or vomiting, allergic dermatitis.
Human reproductive effects by ingestion:
effects on newborn, including Apgar score
changes and other neonatal measures or
effects. Human teratogenic effects by
ingestion: developmental abnormalities of
the cardiovascular system, central nervous
system, musculoskeletal and gastrointestinal
systems. An experimental teratogen.
Experimental reproductive effects.
Experimental carcinogen producing
leukemia and thyroid tumors. Human
mutation data reported. Used in the
treatment of manic-depressive psychoses.
Incompatible with fluorine. See also
LITHIUM COMPOUNDS. |
| Synthesis | Lithium carbonate is synthesized by reacting lithium salts with soda
or potash, followed by purification of the salt, which is not readily soluble [75]. 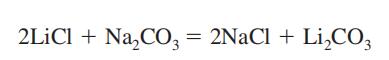
|
| Potential Exposure | Lithium carbonate is used in treatment
of manic-depressive psychoses; to make ceramics and porcelain glaze; varnishes, dyes, pharmaceuticals, coating of
arc-welding electrodes; battery alloys; nucleonics, luminescent paints; glass ceramics; lubricating greases; in aluminum production |
| storage | Store at RT |
| Shipping | UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required |
| Purification Methods | Crystallise it from water. Its solubility decreases as the temperature is raised. The solubility in H2O is 1.3% at ~10o, and 0.7% at ~100o. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963, Caley & Elving Inorg Synth I 1 1939.] |
| Incompatibilities | The aqueous solution is a strong base.
Reacts violently with acids, powdered calcium and fluorine.Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep away
from alkaline materials, strong acids, powdered calcium,
fluorine, moisture. Corrodes aluminum, copper, zinc. |