|
| | Escitalopram oxalate Basic information |
| | Escitalopram oxalate Chemical Properties |
| Melting point | 152-153°C | | alpha | D +12.31° (c = 1 in methanol) | | storage temp. | 2-8°C | | solubility | DMSO: ≥15mg/mL | | form | powder | | color | white to tan | | Merck | 14,2318 | | BCS Class | 1 | | Stability: | Hygroscopic | | InChI | InChI=1S/C20H21FN2O.C2H2O4/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20;3-1(4)2(5)6/h4-9,12H,3,10-11,14H2,1-2H3;(H,3,4)(H,5,6) | | InChIKey | KTGRHKOEFSJQNS-UHFFFAOYSA-N | | SMILES | C1(CCCN(C)C)(C2=CC=C(F)C=C2)C2=C(C=C(C#N)C=C2)CO1.C(O)(=O)C(O)=O | | CAS DataBase Reference | 219861-08-2(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | NP6333500 | | HS Code | 29322090 |
| | Escitalopram oxalate Usage And Synthesis |
| Description | Escitalopram was launched as Cipralex? in Switzerland, Sweden and UK for
the
treatment of depression and panic disorder. It is the S-enantiomer version of the
selective serotonin reuptake inhibitor (SSRI) citalopram approved in 1989. It can be
obtained from 5cyanophthalide by successive reactions with 4-fluorophenyl magnesium
bromide and 3-(dimethylamino)propyl magnesium chloride. The resulting racemic diol can
be resolved by several routes such as crystallization with a chiral acid. Finally, a two step
cyclisation procedure affords escitalopram. Escitalopram is twice as effective as the
racemate and over 100 fold more potent than the R-enantiomer in inhibiting the 5HT
reuptake in vivo in rat brain synaptosomes. Moreover, it exhibits higher selectivity for the
human serotonin transporter relative to the noradrenaline or dopamine transporters than
any other currently available SSRl’s. In the mouse forced swim test, the duration of
immobility (which reflects antidepressant activity) for escitalopram was comparable to
citalopram and greater than (R)-citalopram. Clinical trials in patients with panic disorders or
depression have shown that escitalopram has a clinically relevant and significant effect.
Additionally, it has a faster onset of antidepressant action than citalopram. Escitalopram
has linear pharmacokinetics, with a long half-life (27-32 h). It is extensively metabolized in
the liver via cytochromes P450 to S(+)-desmethyl and S(+)-didesmethyl citalopram.
However, it has been shown to be a weak or negligible inhibitor of CYP450 drugmetabolizing
enzymes in vitro. Escitalopram is well tolerated with nausea being the most
common side effect. | | Chemical Properties | White Solid | | Originator | Lundbeck (Denmark) | | Uses | Escitalopram oxalate may be used as a pharmaceutical primary standard for the quantification of the analyte in pharmaceutical formulations using analytical techniques. | | Uses | An inhibitor of serotonin (5-HT) uptake. Antidepressant | | Uses | A labelled inhibitor of serotonin (5-HT) uptake. Antidepressant | | Uses | antineoplastic | | Brand name | Lexapro (Forest). | | General Description | Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Escitalopram Oxalate, a highly selective serotonin re-uptake inhibitor antidepressant, is a pure S-enantiomer of the racemic, bicyclic pthalates derivatives of citalopram. It is mainly developed for the treatment of depression and anxiety disorders. | | Biochem/physiol Actions | Escitalopram is a selective serotonin reuptake inhibitor (SSRI), the S-enantiomer and eutomer of citalopram. | | Synthesis | The synthesis of escitalopram was carried out in several
different routes [30-33]. 5-Cyanophthalide (72) was treated
with Grignard reagent 73 at 0??C to provide intermediate 75
which was reacted in situ with another Grignard reagent 76
to afford the diol in a one-pot process. Racemic diol 77 was
resolved using (+)-p-toluoyltartaric acid to afford desired S
isomer 78 in 55% yield. The ring closure reaction was
carried out at 0??C using methanesulfonyl chloride in toluene
to furnish escitalopram (7) in 60% yield. 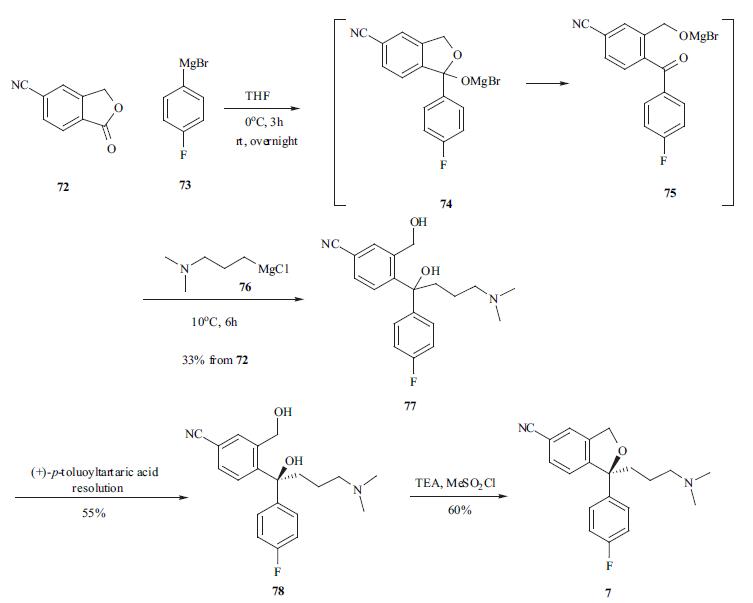
| | storage | +4°C | | references | [1] sánchez c1, bergqvist pb, brennum lt, gupta s, hogg s, larsen a, wiborg o. escitalopram, the s-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. psychopharmacology (berl). 2003 jun;167(4):353-62. epub 2003 apr 26. |
| | Escitalopram oxalate Preparation Products And Raw materials |
|



