|
| Product Name: | Famotidine | | Synonyms: | FAMOTIDINE;[amino-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]-methyl]thio]propylidene]s;3-(((2-((aminoiminomethyl)amino)-4-thiazolyl)methyl)thio)-n-(aminosulfonyl)p;3-(((2-((diaminomethylene)amino)-4-thiazolyl)methyl)thio)-n(sup2)-sulfamoylp;Famotidine USP&BP;FAMOTIDINE, IMP. A (EP) AS HYDROCHLORIDE: 3-[[[2-[DIAMINOMETHYLENE)AMINO]THIAZOL-4-YL]METHYL]-SULPHANYL]PROPANIMIDAMIDE HYDROCHLORIDE MM(CRM STANDARD);FAMOTIDINE, IMPURITY C BP STANDARD;FAMOTIDINE, IMP. B (EP) AS DIMALONATE: 3,5-[2-[[[2-[(DIAMINOMETHYLENE)AMINO]THIAZOL-4-YL]METHYL]-SULPHANYL]ETHYL]-4H-1,2,4,6-THIATRIAZINE 1,1-DIOXIDE DIMALONATE MM(CRM STANDARD) | | CAS: | 76824-35-6 | | MF: | C8H15N7O2S3 | | MW: | 337.45 | | EINECS: | 616-396-9 | | Product Categories: | Other APIs;LODINE;Histamine receptor;Amines;Heterocycles;Sulfur & Selenium Compounds;Intermediates & Fine Chemicals;Pharmaceuticals;API's;76824-35-6 | | Mol File: | 76824-35-6.mol |  |
| | Famotidine Chemical Properties |
| Melting point | 163-164°C | | Boiling point | 562.7±60.0 °C(Predicted) | | density | 1.5111 (rough estimate) | | refractive index | 1.7400 (estimate) | | storage temp. | 2-8°C | | solubility | Very slightly soluble in water, freely soluble in glacial acetic acid, very slightly soluble in anhydrous ethanol, practically insoluble in ethyl acetate. It dissolves in dilute mineral acids | | pka | pKa 6.76(H2O t=23.0) (Uncertain) | | form | neat | | color | White to Off-White | | Water Solubility | 1.1 mg/mL | | BCS Class | 3 | | Stability: | Light Sensitive | | InChIKey | XUFQPHANEAPEMJ-UHFFFAOYSA-N | | CAS DataBase Reference | 76824-35-6(CAS DataBase Reference) | | EPA Substance Registry System | Propanimidamide, 3-[[[2-[(aminoiminomethyl)amino]-4-thiazolyl]methyl]thio]-N-(aminosulfonyl)- (76824-35-6) |
| | Famotidine Usage And Synthesis |
| Description | Famotidine (Chemical formula: C8H15N7O2S3; Brand Name: PEPCID) belongs to a histamine H2-receptor antagonist. It appears as a white to pale yellow crystalline compound. Inside the body, its primary activity is inhibiting the gastric secretion process, further reducing the acid concentration and volume of gastric secretion in the stomach. Based on this property, it is used for the treatment and prevention of ulcers occurring in the stomach and intestines. It can also treat diseases such as Zollinger-Ellison syndrome in which the stomach accumulates excess amount of acids. Moreover, it is also applied during the treatment of gastroesophageal reflux disease (GERD) and pathological hypersecretory conditions.
| | Reference | http://www.rxlist.com/pepcid-drug/clinical-pharmacology.htm
https://en.wikipedia.org/wiki/Famotidine
| | Description | Famotidine is a competitive histamine H2-receptor antagonist, and the main pharmacodynamic
effect of famotidine is to cause the inhibition of gastric secretion. Famotidine on
decomposition releases toxic products such as carbon oxides (CO, CO2), nitrogen oxides (NO, NO2), and sulphur oxides (SO2, SO3). Famotidine is a medication that is available both
in prescription and over-the-counter forms. It is used to treat conditions related to the
oesophagus, stomach, and intestines. Some specific famotidine is used for the treatment of
duodenal ulcers, gastric ulcers (stomach ulcers), gastroesophageal reflux disease (GERD),
and pathological hypersecretory conditions that occur when stomach acid is secreted/
produced in very large quantities, an abnormal health condition called ‘Zollinger-Ellison
syndrome’. | | Chemical Properties | White Powder | | Originator | Yamauouchi (Japan) | | Uses | Histamine H2-receptor antagonist. Antiulcerative. | | Uses | antiinflammatory | | Uses | For the treatment of peptic ulcer disease (PUD) and gastroesophageal reflux disease (GERD). | | Uses | Use as an H2-antagonist. An anti-ulcer agent | | Uses | Contact dermatitis from famotidine, a H2 -receptor
agonist, was described in a nurse. In industry, three
cases were reported due to intermediates of synthesis, 2-
diamino-ethylene-amino-thiazolyl-methylenethiourea-dichloride
and 4-chloromethyl-2-guanidinothiazolenitrochloride.
| | Manufacturing Process | 60.0 kg of dichloroacetone is dissolved in 550 ml of acetone. After cooling the
solution to -5°C, 55.8 kg of amidinothiourea is added to the solution under
cooling portionwise at one hour intervals in a 10 kg amount of
amidinothiourea. The mixture is stirred continuously for 5 days below 0°C.
The 111.6 kg resultant precipitates of N"-[4-(chloromethyl)-4,5-dihydro-4-
hydroxy-2-thiazolyl]-guanidine hydrochloride are collected, and washed with
50 L of acetone. In 500 ml of water are dissolved 111.6 kg of N"-[4-
(chloromethyl)-4,5-dihydro-4-hydroxy-2-thiazolyl]-guanidine hydrochloride
and 32.9 kg of thiourea. The solution is stirred for one hour at 50°C. N'-[4-
[[(Aminoiminomethyl)thio]methyl]-2-thiazolyl]-guanidine dihydrochloride is
formed in the reaction mixture, and this reaction mixture containing this
compound is directly used for the next process without isolation of the formed
compound.
The reaction mixture obtained is cooled below 10°C, and to the solution are
added 45.6 kg of beta-chloropropionitrile and 200 L of isopropanol. A solution
of 69.1 kg of sodium hydroxide in 280 L of water is added dropwise to the
solution under nitrogen stream followed by stirring for 2 hours at 0°C. The
crystals precipitated are collected by filtration, and washed with cold water
and dried to provide 91.7 kg of the N"-[4-[[(2-cyanoethyl)thio]methyl]-2-
thiazolyl]-guanidine, melting point 125-126.5°C.
In 60 L of anhydrous dimethylformamide is dissolved 34.3 kg of the N"-[4-
[[(2-cyanoethyl)thio]methyl]-2-thiazolyl]-guanidine. After adding 60 L of
anhydrous methanol to the solution, 61.9 kg of hydrogen chloride gas is
passed through the solution below 5°C. After stirring the reaction mixture for
2 days at 0°C, the reaction mixture is poured into a mixture of 350 L of water,
250 kg of potassium carbonate, 30 L of ethyl acetate and ice while stirring
below 5°C for 2 hours. The resultant precipitates are collected by filtration.
After stirring a mixture of the precipitates and 400 L of water for 0.5 hour at
0°, the resultant precipitates are collected by filtration, washed with 40 L of
water and 10 L of cooled acetone respectively, and dried at reduced pressure to provide 30.6 kg of the methyl 3-[[[2-[(diaminomethylene)amino]-4-
thiazolyl]methyl]thio]propionimidate showing a melting point of 125.7°C.
In 340 L of methanol is dissolved 88.4 kg of sulfamide under heating, and the
solution is cooled to 30°C. To the solution, 114.2 kg of the methyl 3-[[[2-
[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propionimidate are added
portionwise three times while stirring at 20-30°C. (The second addition is
added 8 hours after the first addition, and the third addition is added 24 hours
after the first addition). After stirring the reaction mixture for a further 2
days, the crystals formed are collected by filtration, washed with 200 L of
cooled methanol, and air-dried at room temperature to provide 87.5 kg of the
3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]-Nsulfamoylpropionamidine
(generic name: famotidine) showing a melting point
of 157.6°C. Some of the obtained product is recrystallized from
dimethylformamide-water, and is dissolved in an equivalent molar amount of
aqueous acetic acid (%). To the solution is added an equivalent molar amount
of a dilute sodium hydroxide solution in water to separate crystals showing a
melting point of 163-164°C. | | Brand name | Fluxid (Schwarz Pharma); Pepcid (Merck);Amifatidine;Famodil;Pepsidac;GASTER. | | Therapeutic Function | Antiulcer | | General Description | Famotidine is a histamine H2-receptor antagonist, which promotes the healing of erosive esophagitis, gastric and duodenal ulcers since it inhibits the gastric acid secretion in humans.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. | | Contact allergens | Contact dermatitis in a nurse from famotidine, an
H2-receptor agonist, was described. In industry, three
cases were reported due to intermediates of the synthesis
of 2-diamino-ethylene-amino-thiazolyl-methylenethio urea-dichloride, and 4-chloromethyl-2-guanidinothiaz ole-nitrochloride. | | Biochem/physiol Actions | H2 histamine receptor antagonist; anti-ulcer agent | | Clinical Use | Famotidine is a histamine H2-antagonist more potent than cimetidine and
ranitidine. Administered once or twice daily, it is useful in the treatment of
gastric, duodenal and anastomotic ulcers, upper gastrointestinal tract hemorrhage,
reflux esophagitis and Zollinger-Ellison syndrome. Like ranitidine, it is lacking in
antiandrogenic effects. | | Synthesis | Famotidine, 3-[[[2-[(aminomethyl)amino]-4-thiazolyl] methyl]thio]-
N-(aminosulfonyl)propanimidamide (16.2.13), is synthesized from S-(2-aminothiazol-4-ylmethyl)
isothiourea (16.2.9), which is synthesized by reacting 1,3-dichloroacetone with two
molecules of thiourea, during which a thiazol ring is formed and the chlorine atom is substituted,
giving an intermediate 2-amino-5-chlormethylthiazol. Reacting this with 2-chlorpropionitrile
gives S-(2-aminothiazol-4-yl-methyl)-2-cyanoethane (16.2.10), which in turn is
reacted with benzoylizthiocyanate. The resulting benzoylthiourea derivative (16.2.11) first
undergoes S-methylation by methyliodide and further cleaved by ammonia into 3-[[[2-
(aminomethyl)amino]-4-thiazolyl]-methyl]thio]ethylcyanide (16.2.12). Successive
methanolysis of the nitrile group and subsequent reaction of the resulting iminoether with
sulfonamide gives famotidine (16.2.13). 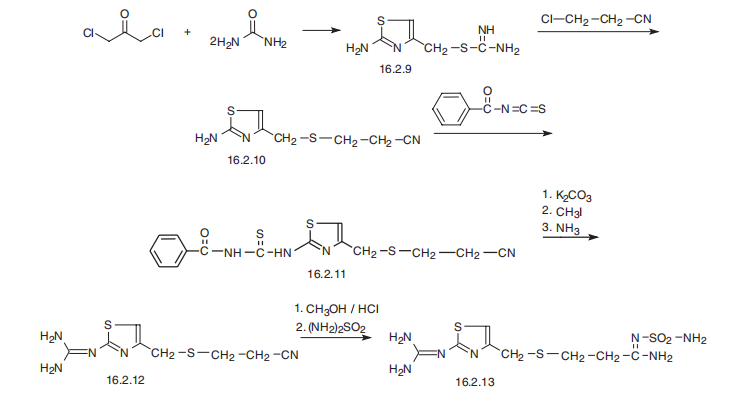
| | Veterinary Drugs and Treatments | In veterinary medicine, famotidine may be useful for the treatment
and/or prophylaxis
of gastric, abomasal and duodenal ulcers,
uremic gastritis, stress-related or drug-induced erosive gastritis,
esophagitis, duodenal gastric reflux, and esophageal reflux.
Famotidine has fewer drug interactions and activity may persist
longer than cimetidine. | | Drug interactions | Potentially hazardous interactions with other drugs
Antifungals: absorption of itraconazole and
ketoconazole reduced; concentration of posaconazole
possibly reduced - avoid with suspension.
Antivirals: concentration of atazanavir reduced
- adjust doses of both drugs; concentration of
raltegravir possibly increased - avoid; avoid for 12
hours before and 4 hours after rilpivirine.
Ciclosporin: possibly increased ciclosporin levels.
Cytotoxics: possibly reduced dasatinib concentration
- avoid if possible; avoid with erlotinib; possibly
reduced absorption of pazopanib - give at least 2
hours before or 10 hours after famotidine; possibly
reduced absorption of lapatinib.
Ulipristal: contraceptive effect possibly reduced -
avoid with high dose ulipristal. | | Metabolism | Metabolism of famotidine occurs in the liver, with
formation of an inactive metabolite, the sulfoxide. Following oral administration, the mean urinary excretion
of famotidine is 65-70% of the absorbed dose, 25-30%
as unchanged compound. Renal clearance is 250-450
mL/min, indicating some tubular excretion. A small
amount may be excreted as the sulfoxide. | | storage | Store at -20°C |
| | Famotidine Preparation Products And Raw materials |
|



