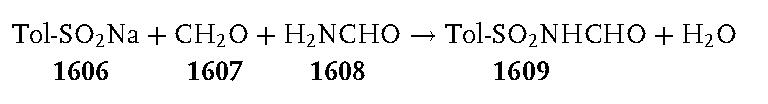|
| | Tosylmethyl isocyanide Basic information |
| | Tosylmethyl isocyanide Chemical Properties |
| Melting point | 109-113 °C(lit.) | | density | 1.2721 (rough estimate) | | refractive index | 1.5270 (estimate) | | storage temp. | 2-8°C | | solubility | water: slightly soluble | | form | Liquid | | color | Clear | | Water Solubility | insoluble | | Sensitive | Moisture Sensitive | | Merck | 14,9556 | | BRN | 3592382 | | Exposure limits | NIOSH: IDLH 25 mg/m3 | | InChI | InChI=1S/C9H9NO2S/c1-8-3-5-9(6-4-8)13(11,12)7-10-2/h3-6H,7H2,1H3 | | InChIKey | BBNNLJMGPASZPD-UHFFFAOYSA-N | | SMILES | S(C1C=CC(C)=CC=1)(=O)(=O)C[N+]#[C-] | | CAS DataBase Reference | 36635-61-7(CAS DataBase Reference) |
| Hazard Codes | T,Xi | | Risk Statements | 23/24/25 | | Safety Statements | 36/37-45-38-36/37/39-28A | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 | | F | 21 | | Hazard Note | Irritant | | HazardClass | 6.1(b) | | PackingGroup | III | | HS Code | 29299000 |
| | Tosylmethyl isocyanide Usage And Synthesis |
| Chemical Properties | Pale yellow to light brown crystalline powder | | Uses | Tosylmethyl Isocyanide is used as a synthetic reagent in the preparation of variety or biologically active heterocycles such as pyrroles and imidazoles. Tosylmethyl Isocyanide is reported to inhibit [
Fe]-hydrogenase with very high affinity. | | Preparation | N-(p-Tolylsulfonylmethyl)formamide 1609:
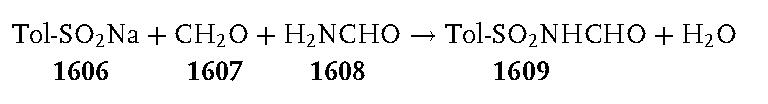
A 3-L, three-necked, round-bottomed flask, equipped with a mechanical stirrer, a condenser, and a thermometer, was charged with sodium p-toluenesulfinate 1606 (267 g, 1.5 mol). After the addition of water (750 mL), a 34–37% solution of formaldehyde 1607 (350 mL, 378 g, ca. 4.4 mol), formamide 1608 (600 mL, 680 g, 15 mol), and formic acid (200 mL, 244 g, 5.3 mol), the stirred reaction mixture was heated at 90 C°. The sodium p-toluenesulfinate dissolved during the heating, and the clear solution was kept at 90–95 C° for 2 h. It was then cooled in an ice/salt bath with continued stirring and further cooled overnight in a freezer at 20 C°. The white solid produced was collected by suction filtration. It was washed thoroughly in a beaker by stirring with three 250 mL portions of iced water. The product was dried under reduced pressure over phosphorus pentoxide at 70 C° to provide 134–150 g (42–47%) of crude N-(p-tolylsulfonylmethyl)formamide 1609; mp 106–110 C°. This product was sufficiently pure to be used directly in the following reaction.
| | Definition | Tosylmethyl isocyanide is a chemical compound of cyanide that Versatile synthon in organic chemistry, especially in the synthesis of heterocyclic Compounds. | | Purification Methods | Use an efficient fume cupboard. Purify TOSMIC by dissolving (50g) in CH2Cl2 (150mL) and passing it through a column (40x3cm) containing neutral alumina (100g) in CH2Cl2 and eluting with CH2Cl2. A nearly colourless solution (700mL) is collected, evaporated in vacuo and the residue (42-47g) of TOSMIC (m 113-114o dec) is recrystallised once from MeOH (m 116-117o dec). [Hoogenboom et al. Org Synth 57 102 1977, Lensen Tetrahedron Lett 2367 1972.] It also crystallises from EtOH (charcoal) [Saito & Itano, J Chem Soc, Perkin Trans 1 1 1986]. |
| | Tosylmethyl isocyanide Preparation Products And Raw materials |
|