|
| | Pirfenidone Chemical Properties |
| Melting point | 96-97°C | | Boiling point | 329.1±15.0 °C(Predicted) | | density | 1.137±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO: ≥10 mg/mL, soluble | | pka | -0.16±0.63(Predicted) | | form | solid | | color | Off-white | | Water Solubility | Soluble to 20 mM in water | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. | | InChIKey | ISWRGOKTTBVCFA-UHFFFAOYSA-N | | CAS DataBase Reference | 53179-13-8(CAS DataBase Reference) |
| | Pirfenidone Usage And Synthesis |
| Overview | Pirfenidone (PFD) is a novel kind of pyridine ketones which has a broad-spectrum anti-fibrotic effect, being able to prevent and reverse the formation of fibrosis and scar. It entered into market in Shionogi, Japan in 2008 and has been approved by the US food and Drug Administration. It is the first drug which has gone through phase III clinical trials (including repeating, randomized, and placebo-control) which has demonstrates its effectiveness in treating idiopathic pulmonary fibrosis (IPF); moreover, the drug has relative good efficacy in treating some fibrotic diseases such as renal interstitial fibrosis and liver fibrosis. Furthermore, it is also widely applied in the phase II clinical study in kidney disease (focal segmental glomerulosclerosis), hypertrophic cardiomyopathy, adult type I neurofibromatosis, youth I type of neurofibromatosis and plexiform neurofibromas, diabetes together with kidney disease.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| | Physical properties | Pirfenidone is a white to pale yellow, non-hygroscopic powder. It is more soluble in methanol, ethyl alcohol, acetone and chloroform than in water and 1.0 N HCl. The melting point is approximately 109°C. | | Uses | Pirfenidone is a broad-spectrum antifibrotic drug that modulates diverse cytokine action involving TGF-β, TNF-α, epidermal growth factor, platelet-derived growth factor,vascular endothelial growth factor, insulin-like growth factor 1, fibroblast growth factor, and others.Pirfenidone has been effective in the prevention and regression of pulmonary fibrosis, peritoneal sclerosis, hepatic cirrhosis, uterine fibromyoma, and other scarring and fibrotic conditions. It doesn’t cure the condition or reverse any existing scarring but it can effectively slow down the rate of lung function decline by about 50%. In the UK, Pirfenidone is currently only approved for treating IPF, and not for other forms of pulmonary fibrosis. | | Preparation | (1) The synthesis of the 5-methyl-2 (1H)-pyridone③
Add 1500 g (13.87 mol) of 2-amino-5-methylpyridine②, 35 L of 5% sulfuric acid to the reaction kettle and stir at room temperature for 15 min. Cool to 0~5 °C after all the solid being totally dissolved. Slowly add 1665 g of sodium nitrite (24.1 mol) into 5 L of water, stir for maintaining temperature at 3h; after the completion of the reaction, heat and reflux until no more gas is generated; use sodium carbonate to adjust pH to neutral, evaporate to dryness under reduced pressure; add 25 L of methanol to the kettle, add 30 g of activated charcoal for heating and reflux for 20min, filter, evaporate the filtrate evaporated to dryness under reduced pressure with the residue being recrystallized by ethanol to obtain pale yellow crystals③.
(2)The Synthesis of pirfenidone ①
Successively add 1000 g (9.16 mol) of 5-methyl-2 (1H)-pyridone, 2245 g (11.01 mol) of iodobenzene, 1391 g (10.08 mol) of potassium carbonate, 32.1 g (0.32 mol) of cuprous chloride and 3.5 L of dimethylsulfoxide into the 10L 3-neck flask, and stir slowly to raise the temperature to 180 °C and maintain the reaction for about 5 h. After the completion of the reaction, cool to room temperature, filter the reaction solution, wash the filter cake with 1 L of dimethyl sulfoxide; combine the filtrate and evaporate to dryness under reduced pressure; add 3 L of 20% acetic acid aqueous solution, and heat to 65 °C; stir for 30 min; stand static for layer separation; further extract the lower oil with 20% aqueous acetic acid (1L X 3) and then combine the aqueous phases. Add solid sodium hydroxide to the aqueous phase to adjust the pH to 13, cool to room temperature, separate out the precipitate; filter; wash the filter cake with water; after drying the filter cake, add 3.2L of ethyl acetate for heating reflux for about 15 min; filter it in hot condition, cool for separate the crystals; allow it to stand overnight; filter; have the filter cake dried under reduced pressure for 5h to obtain the pirfenidone①.

Figure 1 The synthetic route of Pirfenidone (PFD)
This method is a modification of the method 1, which applies the diazotization of 2-amino--5-methylpyridine, hydrolyze to obtain 5-methyl-2 (1H)-pyridone, which have reaction together with bromobenzene in 1: 1.2 (molar ratio); Using DMF as the solvent, CuBr as the catalyst, add 5% of the 1,10-phenanthrolin as the ligand, have reaction at 90°C for 2 hours to obtain the pirfenidone.

Figure 2: The Synthetic route of pirfenidone
| | Pharmacological effects | PFD is a potent inhibitor of a cytokine by regulating or inhibiting certain factors, thus inhibiting the biological activities of fibroblast cells and reducing the cell proliferation and the synthesis of collagen matrix. Meanwhile, PFD can also inhibit the secretion of inflammatory mediators, reduce lipid peroxidation, and exert its anti-inflammatory and antioxidant effects.
1. Inhibition of collagen synthesis
Fibroblast growth factor (bFGF), transforming growth factor-β (transforming growth factor-β, TGF-β), connective tissue growth factor (connective tissue growth factor, CTGF) and tissue inhibitor of metalloproteinase 1 (TIMP-1) are growth factors that related with fibrotic diseases. They can promote fibroblast proliferation and growth, increase the synthesis of collagen matrix and prevent the degradation of extracellular matrix (ECM). They are expressed to different extent during the formation of organ fibrosis. PFD can effectively reduce the expression of the proteins such as bFGF, TGF-β, CTGF and TIMP-1 in the fibroblast cells of lung and kidney and be correlated with the reduction of collagen synthesis and matrix.
The formation of I and III type collagen fibers is also closely related to the synthesis of collagen matrix. At the same time of reducing TGF-β expression, PFD can also reduce the generation α I collagen. PFD also inhibit the expression of pulmonary fibrosis I collagen. In addition, PFD also has inhibitory effect on the expression of type III collagen mRNA and can reduce the synthesis of collagen type III.
2. The anti-inflammatory effects
PFD can inhibit to the inflammatory mediators to varying degrees for exerting their anti-inflammatory effects. When the inflammatory cytokines is overexpressed inside the lung, the inflammation reaction is exacerbated, thus making alveolar wall being thickening, reducing the function of pulmonary alveoli, and ultimately causing fibrosis. PFD primarily take anti-inflammatory effects by inhibiting the expression of inflammatory mediators, reducing vascular permeability, and reducing the agglomeration of neutropenia and inflammatory cells, thus further preventing or slowing down the fibrosis of organs and tissues.
3. Anti-oxidation effect
PFD mainly exert their antioxidant effects by scavenging free radicals and inhibiting lipid peroxidation and oxidative stress mitigation.
| | Pharmacokinetics | After the rat intravenous injection 400 mg• kg-1 of this product, the product is cleared from the plasma according to two-compartment model with a plasma clearance rate being 0.10mL • min-1 • g-1 and the apparent volume of distribution being 0.6 mL • g-1, and the half-life being 8.6min. After intravenous injection, pirfenidone is rapidly distributed in the body fluids with the drug concentration in the tissues reaching peak within 5 min with the concentration among liver, kidneys, lungs and other parts rich in blood containing relative high levels; the concentration in lower fat tissue is relative low; rat: oral administration of pirfenidone: 250~300 mg • kg-1 • d-1 yields a bioavailability of being 25.7%. The 24 h average plasma concentration is (1.9 ± 0.1) μg • mL-1after 14 day of drug administration; 97 % of the metabolites are excreted from the kidney with 24 h excretion accounting for 97% of the total.
| | Clinical application | 1. Idiopathic pulmonary fibrosis degeneration
Idiopathic pulmonary fibrosis (IPF) is an unexplained, poor prognosis and high-mortality chronic inflammatory interstitial lung disease. At the same time, PFD is an anti-inflammatory, anti-fibrotic agent which can be used for the treatment of IPF.
2. Liver Fibrosis
3. Renal fibrosis disease
4. Multiple Sclerosis
Multiple sclerosis (MS) is a central nervous system-related and the immune-related inflammation and demyelinating diseases. Immune factors relating to it are activation of stellate cells, glial cell, and endothelial cell and the increase of lymphocytes, while PDF have effects of inhibiting the cell activation.
5. Myocardial fibrosis
Cardiovascular diseases such as hypertension, cardiomyopathy, and atrial fibrillation can all lead to myocardial fibrosis, thereby resulting in the disease progression or development. Using PDF for treatment of cardiomyopathy caused by Duchenne muscular dystrophy can inhibit myocardial fibrosis, improve the cardiac function. PFD can reduce the incidence of atrial fibrillation and myocardial remodeling through inhibiting fibrosis.
6. Neoplastic diseases: neurofibroma, leiomyoma, and malignant gliomas.
7. Prevention of fibrosis after the organ transplant.
8. Rheumatoid arthritis.
| | Side effects | Common adverse reactions include light sensitivity, fatigue, rash, and nausea; other adverse reactions also include upper gastrointestinal discomfort, bloating, anorexia, diarrhea, and skin itching.
| | Description | Until recently, IPF therapy consisted of a combination of corticosteroids
and immunosuppressive agents (azathioprine and cyclophosphamide)
to target the inflammation that was believed to be the pathogenic
stimulus.Since IPF is now considered to be predominantly a disorder of
fibroproliferation, agents that intervene in fibrogenesis have moved
to the forefront of treatment options. With demonstrated efficacy in a
bleomycin-induced lung fibrosis animal model, pirfenidone has been
developed and launched as an approved therapy for IPF. Its antifibrotic activity is derived from the inhibition of p38 MAP kinase that is
upstream of transforming growth factor-β (TGF-β), a cytokine implicated
in the stimulation of collagen synthesis and the inhibition of its
degradation. Pirfenidone also inhibits the expression of TNF-α, IL-1,
and ICAM-1, so it possesses the dual benefit of an anti-inflammatory and
antifibrotic agent. This relatively simplistic drug is constructed by the copper-catalyzed reaction of 5-methyl-2(1H)-pyridinone with bromo- or chlorobenzene.
. | | Chemical Properties | Off-White Solid | | Originator | Marnac (United States) | | Uses | Pirfenidone has been used:
- as a post-operative eye drop in rabbits to analyse its antifibrotic effect to improve glaucoma filtration surgery
- as an anti-scarring agent to examine whether it affects the foreign body reaction after glaucoma drainage device (GDD) implantation in a rabbit
- to test its antifibrotic potential in primary cultures of human orbital fibroblasts (hOFs)
- as tumor necrosis factor (TNFα) inhibitor to study its effect in hypoxia
| | Uses | Pirfenidone is in a group of medicines called antifibrotic agents. Pirfenidone has demonstrated activity in multiple fibrotic conditions, including those of the lung, kidney and liver. It affects your body’s immune system and reduces the amount of fibrosis (scarring) in the lungs. Pirfenidone is one of two medicines that are approved in Canada to treat Idiopathic Pulmonary Fibrosis (IPF). Another name for this medicine is Esbriet. | | Definition | ChEBI: A pyridone that is 2-pyridone substituted at positions 1 and 5 by phenyl and methyl groups respectively. An anti-inflammatory drug used for the treatment of idiopathic pulmonary fibrosis. | | Brand name | Pirespa | | General Description | Pirfenidone?(5-methyl-1-phenyl-2-[1H]-pyridone) is a synthetic derivative of pyridine. | | Biological Activity | Antifibrotic agent, effective in models of pulmonary and lung fibrosis. Inhibits collagen production and fibroblast proliferation. Regulates cytokine levels following oral administration in vivo . Potent scavenger of free radicals and inhibitor of lipid peroxidation. | | Biochem/physiol Actions | Pirfenidone inhibits collagen production and fibroblast proliferation. It has shown antifibrotic and anti-inflammatory properties in variety of animal models of pulmonary fibrosis, and in clinical trials. | | Synthesis | Pirfenidone
may inhibit collagen synthesis, down-regulate production of
multiple cytokines, and block fibroblast proliferation and stimulation in response to cytokines. Pirfenidone was
prepared via a two step sequence as detailed in the scheme.2-Amino-5-methylpyridine (91) was converted to pyridone
92 by reaction with sulfuric acid and sodium nitrate at
low temperature in 73% yield. Condensation of 5-methyl-2-
(1H)-pyridone (92) with iodobenzene (93) in the presence of
K2CO3 and CuCl at reflux gave pirfenidone (XIII) in 85%
yield. 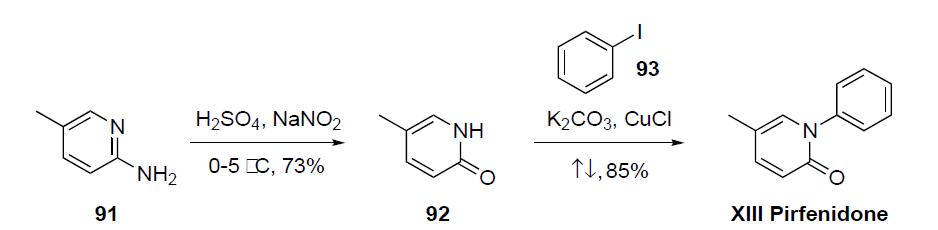
| | in vitro | in raw264.7 cells, pirfenidone (< 300 μg/ml) suppressed the proinflammatory cytokine tumor necrosis factor-α (tnf-α) through a translational mechanism, which was independent of activation of the mitogen-activated protein kinase (mapk)2, p38 map kinase, and c-jun n-terminal kinase (jnk)[1]. in ln-18, t98g, lnt-229 and ln-308 cell lines, pirfenidone (< 10 mm) reduced glioma cell density in a concentration-dependent manner. in ccl-64 cells, pirfenidone (< 5 mm) reduced tgf-β bioactivity by affecting tgf-β2 mrna expression and processing of pro-tgf-β. pirfenidone (< 8.3 mm) inhibited the activity of recombinant furin and downregulated the expression of mmp-11 in a dose-dependent manner in ln-308 cells[2].in cultured myometrial and leiomyoma smooth muscle cells, pirfenidone inhibited serum-stimulated increases in dna synthesis and cell proliferation in a dose-dependent manner[3]. | | in vivo | in animals, pirfenidone treatment significantly decreased gene expression of collagens i, iii and iv, transforming growth factor β-1, smad-7, timp-1 and pai-1 [4]. pirfenidone at a dose of 30 mg/kg/day t.i.d. attenuated the bleomycin-induced pulmonary fibrosi. pirfenidone (30, 100 mg/kg/day t.i.d) suppressed lung inflammatory edema and pulmonary fibrosis. pirfenidone suppressed the bleomycin-induced increase in lung interleukin (il)-1β, il-6, il-12\p40 and monocyte chemoattractant protein (mcp)-1 levels and prevented the bleomycin-induced decrease in lung interferon (ifn)-γ levels. furthermore, pirfenidone suppressed elevation of lung basic-fibroblast growth factor (bfgf), transforming growth factor (tgf)-β1 levels, lung stroma cell derived factor (sdf)-1α and il-18[5]. | | storage | Store at RT | | References | 1) Kehrer and Margolin (1997),?Pirfenidone diminishes cyclophosphamide-induced lung fibrosis in mice; Toxicol.Lett.,?90?125
2) Iyet?et al.?(1999),?Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis; J.Pharmacol.Exp.Ther.,?289?211
3) Xie?et al.?(2002),?Upregulation of RGS2: a new mechanism for pirfenidone amelioration of pulmonary fibrosis; Respir.Res.,?17?103
4) Li?et al.?(2016),?Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-b signaling in a murine colitis model;?Biochem.Pharmacol.,?117?57
5) Nakazato?et al.?(2002),?A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level, Eur.J.Pharmacol.?446?177
6) Misra and Rabideau (2000),?Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals, Mol.Cell Biochem.?204?119
7) Canestaro?et al.?(2016),?Drug Treatment of Idiopathic Pulmonary Fibrosis: Systemic Review and Network Meta-Analysis; Chest,?149?756 |
| | Pirfenidone Preparation Products And Raw materials |
|





