|
| | Mozavaptan Basic information |
| Product Name: | Mozavaptan | | Synonyms: | OPC 31260;MOZAVAPTAN;5-(Dimethylamino)-1-[4-(2-methylbenzamido)benzoyl]-2,3,4,5-tetrahydro-1H-benzazepine;Benzamide, N-[4-[[5-(dimethylamino)-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]carbonyl]phenyl]-2-methyl-;-2,3,4,5-tetrahydro-1H-benzo[b]azepine-1-carbonyl);N-(4-(5-(Dimethylamino);CS-134;OPC-31260; OPC31260L; OPC 31260 | | CAS: | 137975-06-5 | | MF: | C27H29N3O2 | | MW: | 427.54 | | EINECS: | 1312995-182-4 | | Product Categories: | Inhibitors;Pharmaceutical intermediate | | Mol File: | 137975-06-5.mol |  |
| | Mozavaptan Chemical Properties |
| Melting point | 213 - 217°C | | Boiling point | 543.0±50.0 °C(Predicted) | | density | 1.21±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | pka | 13.04±0.70(Predicted) | | form | Solid | | color | White to Off-White |
| | Mozavaptan Usage And Synthesis |
| Description | Mozavaptan is an oral vasopressin V2 antagonist that has been launched in
Japan for inappropriate antidiuretic hormone secretion syndrome (IADHS), an
affliction manifesting as hyponatremia. It joins another nonpeptidic benzazepine,
conivaptan, which corrects sodium and water imbalance by blocking the renal V2
receptor resulting in enhanced diuresis, thereby effectively increasing serum
sodium concentration. While conivaptan inhibits both V1 and V2 receptors,
mozavaptan is significantly more selective for V2 (IC50 of 14nM vs. 1.2 mM for
V1). | | Originator | Otsuka (Japan) | | Uses | Mozavaptan is a vasopressin-receptor antagonist. It is used in the treatment of congestive heart failure and hyponatremia. | | Uses | vasopressin V2 receptor antagonist | | Definition | ChEBI: Mozavaptan is a member of benzamides. | | Brand name | Physuline | | Synthesis | The reported synthesis of mozavaptan is shown in
the scheme. Readily available benzazepin-5-one 39
was refluxed with 40% methyl amine methanol solution
in the presence of molecular sieves for 5h followed by the
reduction of the resulting imine with sodium borohydride to
give the monomethyl amine. Reductive alkylation of the
monomethyl amine with formaldehyde in the presence of
sodium cyanoborohydride gave the dimethyl amino benzazepine
40. Removal of the tosyl group was facilitated by
heating 40 in polyphosphoric acid at 150oC for 2 h to give 41 in 97% yield. Reaction of the resulting benzazepine 41 with
p-nitrobenzoyl chloride (42) in the presence of triethylamine
provided amide 43 which was hydrogenated in the presence
of 10% Pd/C in ethanol at room temperature to give aniline
44. Acylation of aniline 44 with 2-methylbenzoylchloride
(45) in the presence of triethylamine gave mozavaptan (VI)
in 54% yield. 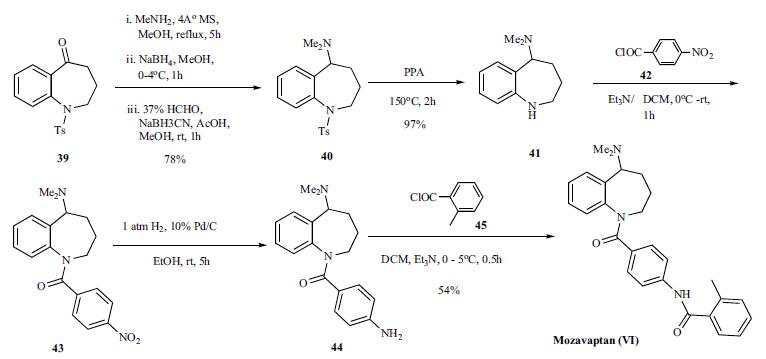
|
| | Mozavaptan Preparation Products And Raw materials |
|