| Chemical Properties | Crystalline Solid |
| Uses | Benzhydroxamic acid is used as precursor in the synthesis of novel mono-anionic and di-anionic hydroxamato complexes by reacting with BiPh3 and Bi(O(t)Bu)3, which has anti-bacterial activity against helicobacter pylori. It is used in the photometric determination of trace amounts of vanadium in alloy steels by making mixed-ligand vanadium chelates with ammonium thiocyanate. It is also involved in the palladium catalyzed synthesis of benzisoxazolones. |
| Uses | Benzhydroxamic acid may be employed for the Pd-catalyzed synthesis of benzisoxazolones. |
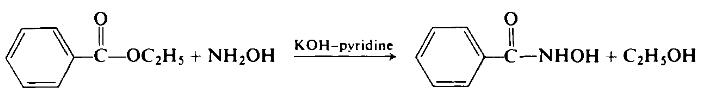
| Preparation | To a rapidly stirred suspension of 19.6 gm (0.35 mole) of powdered anhydrous potassium hydroxide in 120 ml of pyridine, maintained at 0-5°C, is a added a solution of 13.9 gm (0.2 mole) of hydroxylamine hydrochloride in 100 ml of pyridine.
While maintaining the reaction temperature at 0-5°C, 15 gm (0.1 mole) of ethyl benzoate is added. Vigorous stirring is continued at room temperature for 6 hr. Then the solids are filtered off. The solids are washed with cold water to remove inorganic coproducts. The remainder, recrystallized from aqueous ethanol, represents a 94% yield of potassium benzohydroxamate.
This salt is triturated with cold 0.01 TV hydrochloric acid. From this mixture, by the usual procedures, 12.5 gm (91% overall) of benzohydroxamic acid is isolated, m.p. 131°C (from aqueous alcohol).
|
| Synthesis Reference(s) | Tetrahedron Letters, 33, p. 5055, 1992 DOI: 10.1016/S0040-4039(00)61187-5 |
| General Description | Rhombic crystals or light beige solid. |
| Air & Water Reactions | Slightly soluble in water. |
| Reactivity Profile | Benzohydroxamic acid is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). |
| Health Hazard | ACUTE/CHRONIC HAZARDS: When heated to decomposition Benzohydroxamic acid emits toxic fumes of nitrogen oxides. |
| Fire Hazard | Flash point data for Benzohydroxamic acid are not available; however, Benzohydroxamic acid is probably combustible. |
| Safety Profile | Moderately toxic by ingestion.Mutation data reported. When heated to decomposition itemits toxic fumes of NOx. |



