|
| | Unii-33Y9anm545 Basic information |
| Product Name: | Unii-33Y9anm545 | | Synonyms: | Pazopanib HCl;Pazopanib hydrochloride;Unii-33Y9anm545;BenzenesulfonaMide, 5-[[4-[(2,3-diMethyl-2H-indazol-6-yl)MethylaMino]-2-pyriMidinyl]aMino]-2-Methyl-, hydrochloride;786034;ArMala;Pazopanib Hydrochloride (GW786034);5-(4-((2,3-diMethyl-2H-indazol-6-yl)(Methyl)aMino)pyriMidin-2-ylaMino)-2-MethylbenzenesulfonaMide hydrochloride | | CAS: | 635702-64-6 | | MF: | C21H24ClN7O2S | | MW: | 473.98 | | EINECS: | 619-728-0 | | Product Categories: | HFC80011;API | | Mol File: | 635702-64-6.mol |  |
| | Unii-33Y9anm545 Chemical Properties |
| Melting point | >290°C (dec.) | | storage temp. | Hygroscopic, Refrigerator, under inert atmosphere | | solubility | Acetonitrile (Slightly), DMSO (Slightly) | | form | Yellow powder. | | color | White to Off-White | | Stability: | Hygroscopic |
| | Unii-33Y9anm545 Usage And Synthesis |
| Description | The growth of solid tumors is dependent on angiogenesis, the process
wherein new capillaries are formed from existing blood vessels. VEGF is
one of the most important inducers of angiogenesis and expressed at high
levels by most tumors. Hence, the inhibition of VEGF or its receptor
signaling system is an attractive target for cancer therapeutics. The most
studied and developed inhibitors are monoclonal antibodies that neutralize VEGF (e.g., bevacizumab), anti-VEGF ribozymes (e.g., angiozyme),
and small-molecule VEGFR kinase inhibitors (e.g., sunitinib, sorafenib).
Pazopanib is the latest VEGFR kinase inhibitor to reach the market. It is
indicated for the oral treatment of advanced RCC. The biological functions of the VEGF family are mediated by activation of three structurally
homologous tyrosine kinase receptors, VEGFR-1, VEGFR-2, and VEGFR3. In vitro, pazopanib inhibits VEGFR-1, VEGFR-2, and VEGFR-3 with
IC50 values of 10, 30, and 47 nM, respectively.
In addition, it inhibits
several of the closely related tyrosine receptor kinases, including platelet-derived growth-factor receptor β(PDGFR-β), c-kit, and fibroblast
growth factor receptor-1 (FGFR1) with IC50 values of 84, 74, and
140 nM, respectively. In human umbilical vein endothelial cells
(HUVEC), pazopanib inhibits VEGF-induced proliferation more potently
than basic fibroblast growth factor (bFGF)-stimulated proliferation
(IC50 = 21 nM vs. 721 nM) and concentration-dependently inhibits
VEGF-induced VEGFR-2 phosphorylation (IC50 = 7 nM). It also potently
inhibits angiogenesis in Matrigel plug and corneal micropocket assays.
The most common adverse events associated with pazopanib
were diarrhea, hypertension, hair depigmentation, nausea, anorexia,
and vomiting. | | Originator | GlaxoSmithKline (US) | | Uses | Pazopanib Hydrochloride (GW786034, Votrient, Armala) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively. | | Uses | Pazopanib (GW786034) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively - See more at: http://www.selleckchem.com/products/Pazopanib-Hyd | | Uses | The Hydrochloride salt of Pazopanib (P210925) a oral angiogenesis inhibitor targeting VEGFR and PDGFR. | | Definition | ChEBI: A hydrochloride salt prepared from equimolar amounts of pazopanib and hydrochloric acid. Used for treatment of kidney cancer. | | Brand name | Votrient | | Clinical Use | Pazopanib is a potent and selective multi-targeted receptor
tyrosine kinase inhibitor of VEGFR-1, VEGFR-2, VEGFR-3,
PDGFR-a/b, and c-kit that blocks tumor growth and inhibits angiogenesis.
It was approved for renal cell carcinoma by the U.S. Food
and Drug Administration in 2009 and is marketed under the trade
name Votrient by the drug’s manufacturer, GlaxoSmithKline. | | Side effects | Pazopanib is synthesized in five chemical steps
starting from 3-methyl-6-nitroindazole, which is converted to the corresponding
2,3-dimethylindazole analog via N-methylation with trimethyloxonium
tetrafluoroborate. Subsequent reduction of the nitro group to
the amino group using tin chloride followed by condensation with 2,4dichloropyrimidine
yields a chloropyrimidinylaminoindazole intermediate.
The final two steps leading up to pazopanib consist of an N-methylation
reaction using iodomethane and cesium carbonate followed by
condensation with 5-amino-2-methylbenzenesulfonamide. | | Synthesis | The
synthesis of pazopanib begins with methylation of 3-methyl-6-
nitroindazole (82) with trimethyl orthoformate in the presence of BF3?¤OEt to give indazole 83 in 65% yield. Reduction
of the nitro group was achieved via transfer hydrogenation to give
84 in 97% yield, and this was followed by coupling the aniline with
2,4-dichloropyrimidine in a THF-ethanol mixture at elevated
temperature to provide diarylamine 85 in 90% yield. The aniline
nitrogen was then methylated using methyl iodide to give 86 in
83% yield prior to coupling with 5-amino-2-methylbenzenesulfonamide
(87) and salt formation using an alcoholic solution of
HCl to furnish pazopanib hydrochloride (XIV) in 81% yield. 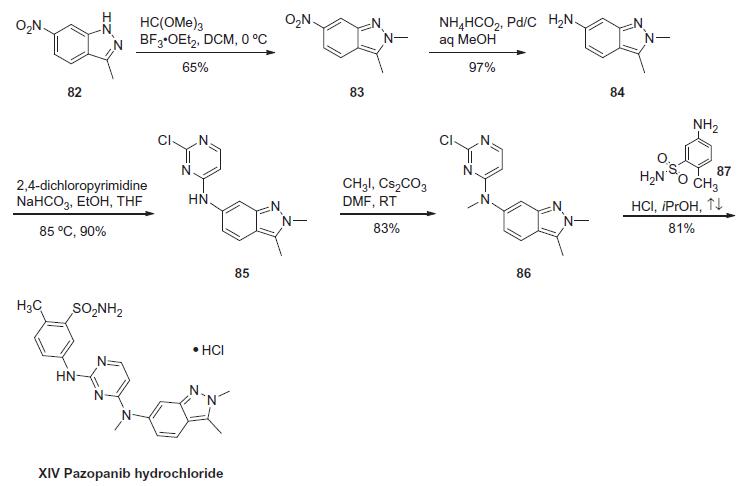
| | target | VEGFR1 |
| | Unii-33Y9anm545 Preparation Products And Raw materials |
|