|
| | Sodium oxide Basic information |
| | Sodium oxide Chemical Properties |
| Melting point | >400°C | | Boiling point | sublimes at 1274℃ [HAW93] | | density | 2.27 g/mL at 25 °C (lit.) | | vapor pressure | 0Pa at 20℃ | | form | Beads | | color | White to gray | | Specific Gravity | 2.27 | | Water Solubility | Soluble in water. | | Sensitive | Air & Moisture Sensitive | | Merck | 14,8651 | | Stability: | Stable. Reacts violently with water, acids and with many other compounds. Store under dry inert gas. May lead to fire in contact with combustible material. | | CAS DataBase Reference | 1313-59-3(CAS DataBase Reference) | | EPA Substance Registry System | Sodium oxide (1313-59-3) |
| | Sodium oxide Usage And Synthesis |
| Chemical Properties | white to grey granules or powder | | Chemical Properties | Sodium is a strong alkaline flux of low toxicity that creates brightly colored glazes. Its exact melting point is 1652°F (900°C) but it begins melting at about 1580°F (860°C), so it's best to begin to reduce high-sodium glazes at this point.
Sodium oxide is a powerful flux at all temperatures. You'll often see it written as KNaO because it's similar to potassium oxide (K2O) in color response and expansion and contraction rate. Often the insoluble source of sodium oxide includes some potassium oxide; for example, feldspars, which are the primary source of sodium in high-fire glazes, usually contain varying amounts of both sodium and potassium oxides.Most sodium oxide sources are soluble and can act as deflocculants in a glaze slurry or a casting slip. Glazes such as copper red or carbon trap shinos,which have soluble sources of sodium oxide like soda ash, borax, ornepheline syenite, can easily become deflocculated.
One interesting characteristic of sodium oxide is that it's volatile above 2012°E (1100°C)and will cause flashing on a clay body as it leaves the glaze. For this rcason it's widely used in such vapor glazing techniques as soda and salt firing.
Sodium exhibits a low viscosity and low surface tension so if it's used in high enough quantities it will cause glazes to run. It also has a high expansion and contraction rate and will often cause crazing in glazes.Sodium oxidc creatcs soft glaze surfaces that are easily abraded by cutlery, acids,and bases (detergents).
Insoluble sources of sodium oxide include sodium feldspars,Minspar, Kona F-4, NC-4, Bainbridge, Eureka, Godfrey, and cryolite. It's also present in potash feldspars, including Custer, G-200, K-200, Plastic Vitrox, Cornwall stone,volanic ash, and rottenstone.Soluble forms of sodium oxide include soda ash, baking soda, salt, borax, unwashed wood as, Gerstley borate and its substitutes, and sodium silicate. Nepheline syenite and most frits are slightly soluble.
| | Uses | Sodium oxide is used in chemical manufacturing, ceramics and glasses. Ethylbenzene dehydrogenation and carbon dioxide shift-reaction occurs using a sodium oxide/alumina catalyst. It is employed as catalyzing gasification of CO2 on carbon. | | Uses | Sodium oxide has been used as a coating precursor on organic polymer films during the plasma modification to analyze its antimicrobial properties. | | Uses | As a dehydrating agent; in certain chemical reactions as a polymerizing or condensing agent. | | Definition | A white solid formed by burning
sodium in a deficiency of oxygen or alternatively by reducing sodium peroxide or
sodium hydroxide with the requisite
amount of sodium. It reacts violently with
water to form sodium hydroxide and with
acids to form solutions of their salts.
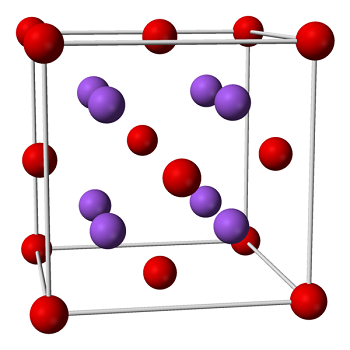
Sodium monoxide forms cubic crystals. It
dissolves in liquid ammonia to give a mixture of sodamide and sodium hydroxide. | | Definition | sodium monoxide: A whitish-greydeliquescent solid, Na2O; r.d. 2.27;sublimes at 1275°C. It is manufacturedby oxidation of the metal in alimited supply of oxygen and purifiedby sublimation. Reaction with waterproduces sodium hydroxide. Its commercialapplications are similar tothose of sodium hydroxide. | | General Description | Sodium oxide is a strong alkaline oxide, commonly used as an active flux in ceramic glazes. | | Flammability and Explosibility | Notclassified |
| | Sodium oxide Preparation Products And Raw materials |
|



