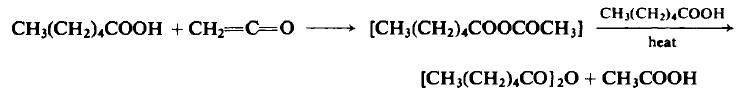| Chemical Properties | clear colorless to light yellow liquid |
| Uses | Hexanoic anhydride was used in:
- green synthesis of esters of acyclovir (acyclovir prodrugs)
- preparation of hexanoyl-modified chitosan nanoparticles
- preparation of chitosan-based polymeric surfactants via N-acylation of chitosans
|
| Uses | Hexanoic anhydride has been used in:
- green synthesis of esters of acyclovir (acyclovir prodrugs)
- preparation of hexanoyl-modified chitosan nanoparticles and chitosan-based polymeric surfactants via N-acylation of chitosans
|
| Uses | Hexanoic Anhydride, is used as a reactant in the total synthesis of acremomannolipin A via steroselective β-mannosylation of 4,6,-O-benzylidene-protected mannosyl sulfoxide with a D-mannitol derivative. |
| Preparation | To an ice-cooled flask containing 116 gm (1.0 mole) of n-caproic acid is added 21.0-23.10 gm (0.5-0.55 mole) of ketene at a rate of 0.45 mole/hr. The reaction mixture is fractionally distilled at atmospheric pressure to afford a forecut of acetone, acetic acid, and acetic anhydride. The oil bath is raised to 220°C over a 1-hr period, kept there for 3 hr to ensure complete removal of acetic acid, and then cooled. The distillation is continued under reduced pressure to afford 86-95 gm (80-87%), b.p. 109- 112°C (3 mm Hg) and b.p. 118-121°C (6 mm Hg).
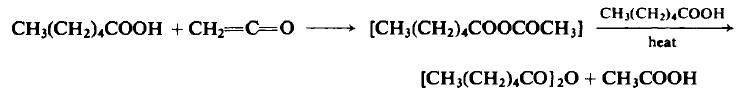
|
| Flammability and Explosibility | Notclassified |