|
| | Azidotrimethylsilane Basic information |
| | Azidotrimethylsilane Chemical Properties |
| Melting point | -95°C | | Boiling point | 92-95 °C(lit.) | | density | 0.876 g/mL at 20 °C(lit.) | | refractive index | n20/D 1.415(lit.) | | Fp | 74 °F | | storage temp. | 2-8°C | | solubility | Miscible with toluene, dichloromethane, diethyl ether and most organic solvents. | | form | Liquid | | color | Clear colorless to slightly yellow | | Specific Gravity | 0.868 | | Water Solubility | decomposes | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | BRN | 1903730 | | InChIKey | SEDZOYHHAIAQIW-UHFFFAOYSA-N | | CAS DataBase Reference | 4648-54-8(CAS DataBase Reference) | | NIST Chemistry Reference | Azidotrimethylsilane(4648-54-8) | | EPA Substance Registry System | Azidotrimethylsilane (4648-54-8) |
| | Azidotrimethylsilane Usage And Synthesis |
| Chemical Properties | clear colorless to slightly yellow liquid | | Physical properties | bp 95–96°C; n20
D 1.416; d 0.868 g cm?3; fp23°C;
IR νmax 2100 cm?1; 1H NMR (CDCl3) δ = 0.22. | | Uses | Versatile azidonation reagent for amines, amides, aldehydes, and ketones. | | Uses |
Many applications of TMSA in organic synthesis have been reported but only representative examples are described herein.
Substitution Reactions.
Benzyl,allyl,and substituted alkyl halides are converted to the corresponding azides in 60–100% yields via reactions with TMSA under neutral conditions in a nonaqueous solvent (eq 1).By using tin(IV) chloride as a catalyst, secondary and tertiary cyclic and polycyclic halides are similarly transformed into the corresponding azides in 50–92% yields (eq 1).
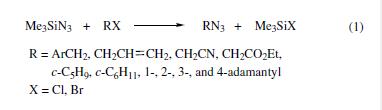
| | Uses | Trimethylsilyl azide is used as an azidonation reagent for amines, amides, aldehydes and ketones. It is involved in the preparation of alfa- and beta-siloxy azides from carbonyl compounds and epoxides. It is also used in the preparation of heterocyclic compounds and also acts as an effective substitute for hydrazoic acid. Further, it is used in the Oseltamivir total synthesis. | | Preparation | several methods for the synthesis of this
azide have been reported.The procedure involving aluminum
chloride is not recommended, since an explosive product is
formed.Azidotrimethylsilane is now commercially available,
and a representative synthetic procedure is as follows. A mixture
of sodium azide and chlorotrimethylsilane is refluxed in
di-n-butyl ether for 2 days and the azide is safely distilled directly
from the reaction vessel. Purer compound (99% content)
is obtained by redistillation of the product. Several improved
conditions have been reported for the preparation of this
azide.In these procedures, trimethylsilyl chloride is reacted
with sodium azide either neat or in a high boiling point solvent,
such as a mixture of silicone oil and polyethylene glycol.
Distillation of the crude product usually provides trimethylsilyl
azide (TMSA) in high purity (97.9%) and yield (97%). | | Purification Methods | Distil the azide through a Vigreux column (p 11) in a N2 atmosphere maintaining the oil bath temperature thermostat at 135-140o . Check the purity by 1H NMR [CHCl3, : single peak at 13cps from Me4Si]. Likely impurities are siloxane hydrolysis products. The azide is thermally stable even at 200o when it decomposes slowly without explosive violence. All the same, it is advisable to carry out the distillation behind a thick safety screen in a fumehood because unforseen EXPLOSIVE azides may be formed on long standing. [Birkofer & Wagner Org Synth Coll Vol VI 1030 1988.] |
| | Azidotrimethylsilane Preparation Products And Raw materials |
|



